

Chinese Journal of Organic Chemistry >
A Novel Triad Fluorescent Chemodosimeter with 4,5-Bis(2’-cyano-ethylthio)-1,3-dithiole-2-thione and Anthracene Units for Hg(II) Recognition and Its Fluorescence Properties in Ionic Liquid
Received date: 2013-01-02
Revised date: 2013-02-05
Online published: 2013-03-07
Supported by
Project supported by Natural Science Foundation of Anhui Province Institution of Higher Learning (No. KJ2010A307) and the Foundation of Science and Technology Talent of Huaibei City (No.20120310).
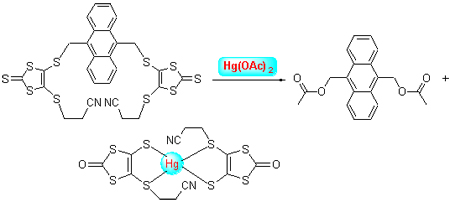
4,5-Bis(2'-cyanoethylthio)-1,3-dithiole-2-thione (DMIT) was treated with sodium methoxide to deprotect one 2-cyanoethyl group, affording mono-thiolate of DMIT. The generated mono-thiolate was trapped by 9,10-dicholormethylene anthracene to yield a novel triad fluorescent chemodosimeter with DMIT and anthracene units. The triad reacted with Hg(OAc)2 to yield a strongly fluorescent compound 4 and a complex 5 of DMIT. So the triad could selectively recognize Hg2+ by the unique properties of compound 4. Fluorescence properties of the triad were further explored in ionic liquid. The experiment results showed that fluorescence intensity of the triad became more strengthen with amounts of ionic liquid.
Key words: 1,3-dithiole-2-thione; fluorescent chemodosimeter; synthesis; ionic liquid
Chi Xingbao , Liu Yang . A Novel Triad Fluorescent Chemodosimeter with 4,5-Bis(2’-cyano-ethylthio)-1,3-dithiole-2-thione and Anthracene Units for Hg(II) Recognition and Its Fluorescence Properties in Ionic Liquid[J]. Chinese Journal of Organic Chemistry, 2013 , 33(07) : 1545 -1550 . DOI: 10.6023/cjoc201301002
/
| 〈 |
|
〉 |