

Chinese Journal of Organic Chemistry >
Synthesis and Characterization of Dihydrazide Derivatives Containing Benzimidazole Ring
Received date: 2013-01-04
Revised date: 2013-02-26
Online published: 2013-03-07
Supported by
Project supported by the Natural Science Foundation of Liaoning Province (No. 20102126).
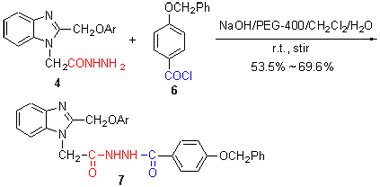
Ten novel dihydrazide derivatives containing benzimidazole ring (7a~7j) were synthesized by (un)substituted phenol and chloroacetic acid as starting materials via a series of reactions. The structure of representative compound 7c was confirmed by 1H NMR, 13C NMR, 2D NMR (1H-1H COSY, HSQC, HMBC and NOESY) and variable-temperature 1H NMR spectra. The experimental results indicate that compound 7c in DMSO at room temperature exists in the tautomeric A and B isomers, and A isomer is dominant (87.65%). On the basis of the analytic results of compound 7c, 1H NMR spectra of other target compounds were also assigned and the content of dominant A isomer is in the range of 80.55%~89.90%.
Key words: dihydrazide; synthesis; characterization
Li Yingjun , Yu Yang , Xu Yongting , Jin Kun , Luo Tongchuan , Shao Xin , Shi Xiangling , Wu Jianghong . Synthesis and Characterization of Dihydrazide Derivatives Containing Benzimidazole Ring[J]. Chinese Journal of Organic Chemistry, 2013 , 33(07) : 1551 -1558 . DOI: 10.6023/cjoc201301009
/
| 〈 |
|
〉 |