

Chinese Journal of Organic Chemistry >
Synthesis Antimicrobial Activities and Side Reactions of 4-Methyl-3-acetyl-2-heterocyclic-2H,3H-benzo[f]-1,5-thiazepines
Received date: 2012-12-23
Revised date: 2013-02-08
Online published: 2013-03-14
Supported by
Project supported by the National Natural Science Foundation of China (No. 20972040).
Starting from substituted heterocyclic aldehyde and acetylacetone, a series of new 4-methyl-3-acetyl-2-heterocyc-lic-2H,3H-benzo[f]-1,5-thiazepines 3a~3l were synthesized through 3-step reactions. The structures of the new products were confirmed by 1H NMR, IR, MS and elemental analysis, and the imine-enamine tautomerism of the target compouds was investigated by means of 1H NMR spectroscopy. Their antifungal activities were evaluated, and the results provides the inspiration for structure-activity reationship of the benzothiazepines. Meanwhile, the structure of the main by-products of 3h was identified, and the possible mechanism of the by-products was proposed.
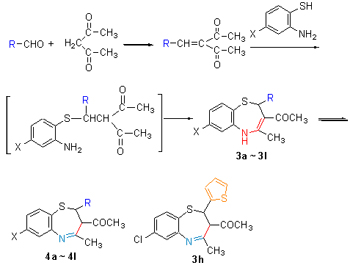
Key words: 1,5-benzothiazepine; heterocyclic; side reaction; antifungal activity
Wu Yunyun , Tian Keqing , Yang Tian , Du Xingqiong , Zhang Ping . Synthesis Antimicrobial Activities and Side Reactions of 4-Methyl-3-acetyl-2-heterocyclic-2H,3H-benzo[f]-1,5-thiazepines[J]. Chinese Journal of Organic Chemistry, 2013 , 33(07) : 1465 -1471 . DOI: 10.6023/cjoc201212027
/
| 〈 |
|
〉 |