

Chinese Journal of Organic Chemistry >
Solvent-Free Synthesis of 5-Alkenyl-2,2-pentamethylene-1,3-dioxane-4,6-diones under Ultrasonic Irradiation with CePW12O40 as Catalyst
Received date: 2012-12-23
Revised date: 2013-03-06
Online published: 2013-03-14
Supported by
Project supported by the National Science and Technology Project (No. 2001BA323C) and the Graduate Innovation Fundation of Jiangxi Province (No. YC10A051).
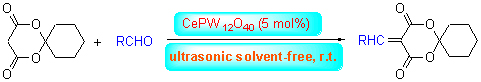
Eight kinds of 5-alkenyl-2,2-pentamethylene-1,3-dioxane-4,6-diones were synthesized by the Knoevenagel condensation reaction of aromatic aldehydes with 2,2-pentamethylene-1,3-dioxane-4,6-dione using CePW12O40 as catalyst, without solvent under ultrasonic irradiation. The results indicate that the yields ranged from 87.6% to 94.1% when using 5% (molar fraction) CePW12O40 and reacting at room temperature for 20~35 min. Furthermore, a proposed reaction mechanism for the reaction catalyzed by CePW12O40 was speculated. Compared to the classical Knoevenagel condensation reaction, the main advantages of the present procedure were milder conditions, shorter reaction time and higher yields, which afforded an effective method to synthesize 5-alkenyl-isopropylidene malonate derivatines. Further study showed that CePW12O40 was environmentally friendly and reused for four times without any noticeable decrease in the catalytic activity.
Xu Zhaohui , Lin Chunhua . Solvent-Free Synthesis of 5-Alkenyl-2,2-pentamethylene-1,3-dioxane-4,6-diones under Ultrasonic Irradiation with CePW12O40 as Catalyst[J]. Chinese Journal of Organic Chemistry, 2013 , 33(07) : 1540 -1544 . DOI: 10.6023/cjoc201212039
/
| 〈 |
|
〉 |