

Chinese Journal of Organic Chemistry >
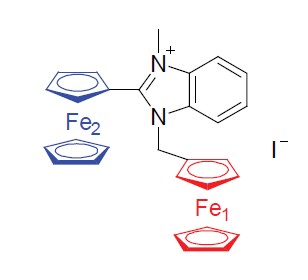
Synthesis Crystal Structure and Properties of Iodide, Hexafluoro- phosphate and Bis(trifluoromethane sulfonamide) Salts of 1-Ferrocenyl Methyl-2-ferrocenyl-3-alklybenzimidazolium
Received date: 2013-01-15
Revised date: 2013-03-01
Online published: 2013-03-14
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21202019, 21172036), the National Science Foundation for Fostering Talents in Basic Research of China (No. J1103303) and the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry (No. [2011]1568).
1-Ferrocenly methyl-2-ferrocenly benzimidazole was sythesized by the reaction of o-phenylenediamine with ferrocenecarboxaldehyde in the condition of toluenesulfonic acid as catalyst. Iodized salts (3, 4) of 1-ferrocenyl methyl- 2-ferrocenyl-3-alkylbenzimidazolium were formed by the reaction of compound 2 with alkyl iodide. Hexafluorophosphate salts (5, 6) and bis(trifluoromethane sulfonimide) salts (7, 8) of 1-ferrocenyl methyl-2-ferrocenyl-3-alkylbenzimidazolium were obtained by the anion exchange reaction of compounds 3 and 4. All of these salts were characterized by 1H NMR, 13C NMR, IR, MS, HRMS and elemental analysis. In the crystal of compound 5, the compound was assembled to a chain supramolecular structure along axis c by the intermolecular hydrogen bonds. Electrochemical analysis demonstrated that the quasi-reversible oxidation waves split double of Fe in ferrocene fragment of compounds 3~8; UV-Vis absorption spectra show that these salts possess photoinduced charge migration phenomenon. And the iodized salts presented good catalytic effect on decomposition of ammonium perchlorate (AP) in DSC-TG test.
Ye Hongmin , Wang Wei , Zhu Xiaoxiao , Chen Weiqiang , Xie Lili , Yuan Yaofeng . Synthesis Crystal Structure and Properties of Iodide, Hexafluoro- phosphate and Bis(trifluoromethane sulfonamide) Salts of 1-Ferrocenyl Methyl-2-ferrocenyl-3-alklybenzimidazolium[J]. Chinese Journal of Organic Chemistry, 2013 , 33(04) : 827 -834 . DOI: 10.6023/cjoc201301036
/
| 〈 |
|
〉 |