

Chinese Journal of Organic Chemistry >
Synthesis and Photoelectrical Properties of 5,10,15,20-Tetra{4-[(N-carbazyl)butyloxyphenyl]}porphyrin and Its Transition Metal Complexes
Received date: 2013-01-29
Revised date: 2013-03-15
Online published: 2013-03-22
Supported by
Project supported by the National Natural Science Foundation of China (No. 20801022).
A novel 5,10,15,20-tetra{4-[(N-carbazyl)butyloxyphenyl]}porphyrin and its transition metal complexes[M=Co (2), Ni (3), Cu (4), Zn (5)] were prepared and characterized by UV-Vis, 1H NMR, IR, Raman spectra and elemental analyses. Compared with the porphyrin ligand, the number of the absorption bands of the metalloporphyrin complexes decrease, the most remarkable difference was the absence of some Q bands. When the metal ions substituted the protons on the N atoms in pyrrole rings, the symmetry of the molecule was changed from D2h to D4h, therefore their absorption spectra were changed to some extent. The IR bands at 3315 and 966 cm-1 in the free base porphyrin ligand are assigned to the N—H stretching and bending vibration of the porphyrin core, respectively. The N—H stretching and bending vibration disappear in the metallporphyrin complexes, since hydrogen atom in N—H bonding is replaced by transition metal ion. There were high fluorescence intensity and fluorescence quantum yield of the porphyrin ligand and its zinc complex. Fluorescence quantum yield of porphyrin zinc complex was higher than that of ZnTPP. The fluorescence intensity of porphyrin nickel complex was quenched partly but the fluorescence intensity of porphyrin cobalt complex and porphyrin copper complex were quenched completely. This indicates fairly certainly that the spin forbidden process S1~→Tn is predominant for radiationless deactivation of S1 in porphyrin compounds. Contrary to the results of the fluorescence intensity of porphyrin complexes, there were high surface photovoltage of porphyrin cobalt complex and porphyrin copper complex without external field. Competition process was showed between fluorescence intensity and surface photovoltage intensity. Positive external field could increase surface photovoltage intensity of porphyrin complexes. Surface photovoltage spectra of porphyrin complexes were influence by the property of external electric field which indicated that the porphyrin complexes were probably used for photovoltaic materials.
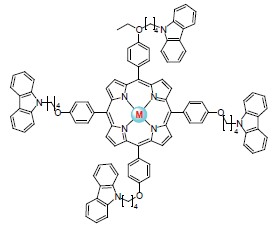
Key words: porphyrin; transition metal; complex; synthesis; fluorescence; surface photovoltage
Wang Binbin , Shan Ning , Xia Aiqing , Wang Yunfang , Yu Miao , Shi Tongshun . Synthesis and Photoelectrical Properties of 5,10,15,20-Tetra{4-[(N-carbazyl)butyloxyphenyl]}porphyrin and Its Transition Metal Complexes[J]. Chinese Journal of Organic Chemistry, 2013 , 33(08) : 1810 -1816 . DOI: 10.6023/cjoc201301074
/
| 〈 |
|
〉 |