

Chinese Journal of Organic Chemistry >
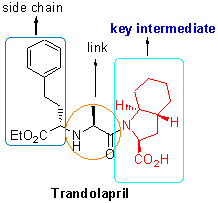
Synthesis of (2S,3aR,7aS)-Benzyl Octahydro-1H-indole-2-carboxylate
Received date: 2013-02-02
Revised date: 2013-02-21
Online published: 2013-02-22
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21172143, 21172145, 21232004).
The key intermediate of antihypertensive drugs trandolapril, (2S,3aR,7aS)-benzyl octahydro-1H-indole-2-car-boxylate, was synthesized by improvement of synthetic route and optimation of synthetic technology with satisfactory results (99% ee and 13.2% overall yield). The structure and absolute configuration of the product were characterized by NMR and HPLC analysis, which are completely consistent with the reported data of the literature. It was obvious that the current methodology provided an efficient pathway for the synthesis of trandolapril.
Shi Tao , Shen Jiefeng , An Qianjin , Liu Delong , Liu Yangang , Zhang Wanbin . Synthesis of (2S,3aR,7aS)-Benzyl Octahydro-1H-indole-2-carboxylate[J]. Chinese Journal of Organic Chemistry, 2013 , 33(07) : 1573 -1577 . DOI: 10.6023/cjoc201302006
/
| 〈 |
|
〉 |