

Chinese Journal of Organic Chemistry >
Research Progress in Friedel-Crafts Reaction of Indoles and Nitroalkenes
Received date: 2013-01-21
Revised date: 2013-03-18
Online published: 2013-04-07
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21262034, 20962018, 20862015, 20562011)
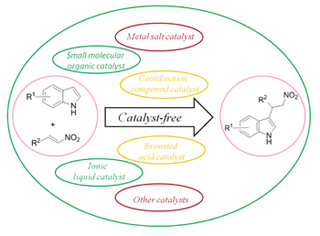
Indoles, as the core structure, exist widely in many alkaloid natural products and pharmaceuticals. The derivatives of indole play an important role in organism physiological activities. The Friedel-Crafts reaction of indoles and nitroalkenes is an important method to generate indole derivatives. According to the kinds of the catalyst in the reaction, the Friedel-Crafts reactions of indoles and nitroalkenes were summarized in this paper as follows: (1) catalyst-free, (2) ionic liquid catalyst, (3) metal salt catalyst, (4) coordination compound catalyst, (5) Brønsted acid catalyst, (6) small molecular organic catalyst, (7) other catalysts.
Key words: Friedel-Crafts reaction; indole; nitroolefin; catalyst
Wang Chunchao , Xie Shaolei , Xie Zhengfeng . Research Progress in Friedel-Crafts Reaction of Indoles and Nitroalkenes[J]. Chinese Journal of Organic Chemistry, 2013 , 33(9) : 1919 -1931 . DOI: 10.6023/cjoc201301051
[1] Sartori, G.; Maggi, R. Chem. Rev. 2011, 111, 181.
[2] Zhuang, W.; Gathergood, N.; Hazell, R. G.; Jørgensen, K. A. J. Org. Chem. 2001, 66, 1009.
[3] (a) Choudhury, J.; Podder, S.; Roy, S. J. Am. Chem. Soc. 2005, 127, 6162.
(b) Firouzabadi, H.; Iranpoor, N.; Nowrouzi, F. Tetrahedron Lett. 2003, 44, 5343.
(c) Cardoso, L. A. M.; Jr, W. A.; Gonzaga, A. R. E.; Aguiar, L. M. G.; Andrade, H. M. C. J. Mol. Catal. A: Chem. 2004, 209, 189.
(d) Huang, J. W.; Shi, M. Tetrahedron Lett. 2003, 44, 9343.
(e) Kuwabara, J.; Takeuchi, D.; Osakada, K. J. Mol. Catal. A: Chem. 2004, 208, 39.
(f) Zhou, Y.; Li, X.; Hou, S.; Xu, J. J. Mol. Catal. A: Chem. 2012, 365, 203.
[4] (a) Boon, J. A.; Levisky, J. A.; Pflug, J. L.; Wilkes, J. S. J. Org. Chem. 1986, 51, 480.
(b) Moore, R. E.; Cheuk, C.; Yang, X. Q. G.; Patterson, G. M. L. J. Org. Chem. 1987, 52, 1036.
(c) Dalpozzo, R.; Bartoli, G.; Bencivenni, G. Chem. Soc. Rev. 2012, 41, 7247.
(d) Miller, K. A.; Williams, R. M. Chem. Soc. Rev. 2009, 38, 3160.
[5] (a) Fukuyama, T.; Chen, X. J. Am. Chem. Soc. 1994, 116, 3125.
(b) Moore, R. E.; Cheuk, C.; Patterson, G. M. L. J. Am. Chem. Soc. 1984, 106, 6456.
(c) Chen, Y.-C.; Xie, Z.-F. Chin. J. Org. Chem. 2012, 32, 462 (in Chinese).
(陈永诚, 解正峰, 有机化学, 2012, 32, 462.)
[6] Noland, W. E. Chem. Rev. 1954, 137.
[7] Suter, W.; Hartmann, A.; Poetter, F.; Sagelsdorff, P.; Hoffmann, P.; Martus, H. J. Mater. Res. 2002, 518, 181.
[8] (a) Hudlicky, T.; Entwistle, D. A.; Pitzer, K. K.; Thorpe, A. J. Chem. Rev. 1996, 96, 1195.
(b) Corey, E. J.; Zhang, F. Y. Angew. Chem., Int. Ed. 1999, 38, 1931.
(c) Kieß, F. M.; Poggendorf, P.; Picasso, S.; Jäger, V. Chem. Commun. 1998, 119.
(d) Shiri, M. Chem. Rev. 2012, 112, 3508.
(e) Shiri, M.; Zolfigol, M. A.; Kruger, H. G.; Tanbakouchian, Z. Chem. Rev. 2010, 110, 2250.
[9] Jeon, S. J.; Li, H.; Walsh, P. J. J. Am. Chem. Soc. 2005, 127, 16416.
[10] Habib, P. M.; Kavala, V.; Kuo, C. W.; Yao, C. F. Tetrahedron Lett. 2008, 49, 7005.
[11] Gu, Y.; Barrault, J.; Jérôme F. Adv. Synth. Catal. 2008, 350, 2007.
[12] Habib, P. M.; Kavala, V.; Raju, B. R.; Kuo, C. W.; Huang, W. C.; Yao, C. F. Eur. J. Org. Chem. 2009, 4503.
[13] Rosa, M. D.; Soriente, A. Tetrahedron 2010, 66, 2981.
[14] Habib, P. M.; Kavala, V.; Kuo, C. W.; Raihan, M. J.; Yao, C. F. Tetrahedron 2010, 66, 7050.
[15] Fu, Z.; Shao, H. Ultrason. Sonochem. 2011, 18, 520.
[16] (a) Sheldon, R. Chem. Commun. 2001, 2399.
(b) Dupont, J.; Souza, R. F.; Suarez, P. A. Z. Chem. Rev. 2002, 102, 3667.
(c) Stolte, S.; Abdulkarim, S.; Arning, J.; Blomeyer-Nienstedt, A. K.; Bottin-Weber, U.; Matzke, M.; Ranke, J.; Jastorff, B.; Thöming, J. Green Chem. 2008, 10, 214.
(d) Hasegawa, E.; Hiroi, N.; Osawa, C.; Tayama, E.; Iwamoto, H. Tetrahedron Lett. 2010, 51, 6535.
[17] Yadav, J. S.; Reddy, B. V. S.; Baishya, G.; Reddy, K. V.; Narsaiah, A. V. Tetrahedron 2005, 61, 9541.
[18] Lin, J. H.; Zhang, C. P.; Zhu, Z. Q.; Chen, Q. Y.; Xiao, J. C. J. Fluorine Chem. 2009, 130, 394.
[19] Shen, W.; Wang, L.; Tang, J.; Qian, Z.; Tong, X. Chin. J. Chem. 2010, 28, 443.
[20] Poulsen, T. B.; Jørgensen, K. A. Chem. Rev. 2008, 108, 2903.
[21] Ballini, R.; Gabrielli, S.; Palmieri, A.; Petrini, M. Tetrahedron 2008, 64, 5435.
[22] Tu, Z.; Raju, B. R.; Liou, T. R.; Kavala, V.; Kuo, C. W.; Jang, Y.; Shih, Y. H.; Wang, C. C.; Yao,C. F. Tetrahedron 2009, 65, 2436.
[23] Zahouily, M.; Abrouki, Y.; Rayadh, A.; Sebti, S.; Dhimane, H.; David, M. Tetrahedron. Lett. 2003, 44, 2463.
[24] An, L.; Zhang, L.; Zou, L. J. Chin. J. Chem. 2009, 27, 2223.
[25] Meshram, H. M.; Kumar, D. A.; Reddy, B. C. HeIv. Chim. Acta 2009, 92, 1002.
[26] Schwalm, C. S.; Ceschi, M. A.; Russowsky, D. J. Braz. Chem. Soc. 2011, 22, 623.
[27] (a) Guo, F.; Lai, G.; Xiong, S.; Wang, S.; Wang, Z. Chem. Eur. J. 2010, 16, 6438.
(b) Nakamura, A.; Lectard, S.; Hashizume, D.; Hamashima, Y.; Sodeoka, M. J. Am. Chem. Soc. 2010, 132, 4036.
[28] Huang, G.; Sun, H.; Qiu, X.; Shen, Y.; Jiang, J.; Wang, L. J. Organomet. Chem. 2011, 696, 2949.
[29] Wan, N. N.; Yang, Y. L.; Wang, W. P.; Xie, Z. F.; Wang, J. D. Chin. Chem. Lett. 2011, 22, 1155.
[30] Wan, N.; Hui, Y.; Xie, Z.; Wang, J. Chin. J. Chem. 2012, 30, 311.
[31] Jia, Y. X.; Zhu, S. F.; Zhou, Q. L. J. Org. Chem. 2006, 71, 75.
[32] Singh, P. K.; Bisai, A.; Singh, V. K. Tetrahedron Lett. 2007, 48, 1127.
[33] Itoh, J.; Fuchibe, K.; Akiyama, T. Angew. Chem., Int. Ed. 2008, 47, 4016.
[34] Liu, H.; Lu, S. F.; Xu, J.; Du, D. M. Chem. Asian J. 2008, 3, 1111.
[35] McKeon, S. C.; Müller-Bunz, H.; Guiry, P. J. Eur. J. Org. Chem. 2009, 4833.
[36] Cattoën, X.; Pericàs, M. A. Tetrahedron 2009, 65, 8199.
[37] Wu, L. Y.; Hao, X. Q.; Xu, Y. X.; Jia, M. Q.; Wang, Y. N.; Gong, J. F.; Song, M. P. Organometallics 2009, 28, 3369.
[38] Kim, H Y.; Kim, S.; Oh, K. Angew. Chem. Int. Ed. 2010, 49, 4476.
[39] Liu, H.; Du, D. M. Adv. Synth. Catal. 2010, 352, 1113.
[40] Wu, J.; Li, X.; Wu, F.; Wan, B. Org. Lett. 2011, 13, 4834.
[41] McKeon, S. C.; Müller-Bunz, H.; Guiry, P. J. Eur. J. Org. Chem. 2011, 7107.
[42] Peng, J.; Du, D. M. Eur. J. Org. Chem. 2012, 4042.
[43] (a) Akiyama, T.; Morita, H.; Itoh, J.; Fuchibe, K. Org. Lett. 2005, 7, 2583.
(b) Wabnitz, T. C.; Spencer, J. B. Org. Lett. 2003, 5, 2141.
(c) Uraguchi, D.; Terada, M. J. Am. Chem. Soc. 2004, 126, 5356.
(d) Li, Z.; Zhang, J.; Brouwer, C.; Yang, C. G.; Reich, N. W.; He, C. Org. Lett. 2006, 8, 4275.
(e) Niu, R.; Xiao, J.; Liang, T.; Li, X.; Org. Lett. 2012, 14, 676.
[44] An, L. T.; Zou, J. P.; Zhang, L. L.; Zhang, Y. Tetrahedron Lett. 2007, 48, 4297.
[45] Sheng, Y. F.; Li, G. Q.; Kang, Q.; Zhang, A. J.; You, S. L. Chem. Eur. J. 2009, 15, 3351.
[46] Zheng, C.; Sheng,Y. F.; Li, Y. X. You, S. L. Tetrahedron 2010, 66, 2875.
[47] Li, N.; Chen, X. H.; Zhou, S. M.; Luo, S. W.; Song, J.; Ren, L.; Gong, L. Z. Angew. Chem., Int. Ed. 2010, 49, 6378.
[48] Marqués-López, E.; Alcaine, A.; Tejero, T.; Herrera, R. P. Eur. J. Org. Chem. 2011, 3700.
[49] (a) Kumar, V. P.; Sridhar, R.; Srinivas, B.; Narender, M.; Rao, K. R. Can. J. Chem. 2008, 86, 907.
(b) Ganesh, M.; Seidel, D. J. Am. Chem. Soc. 2008, 130, 16464.
[50] Kuo, C. W.; Wang, C. C.; Fang, H. L.; Raju, B. R.; Kavala, V.; Habib, P. M.; Yao, C. F. Molecules 2009, 14, 3954.
[51] Schafer, A. G.; Wieting, J. M.; Mattson, A. E. Org. Lett. 2011, 13, 5228.
[52] Tran, N. T.; Wilson, S. O.; Franz, A. K. Org. Lett. 2012, 4, 186.
[53] Herrera, R. P.; Sgarzani, V.; Bernardi, L.; Ricci, A. Angew. Chem., Int. Ed. 2005, 44, 6576.
[54] Zhuang, V.; Hazell, R. G.; Jørgensen, K. A. Org. Biomol. Chem. 2005, 3, 2566.
[55] Bui, T.; Syed, S.; Barbas, C. F. J. Am. Chem. Soc. 2009, 131, 8758.
[56] Schneider, J. F.; Falk, F. C.; Fröhlich, R.; Paradies, J. Eur. J. Org. Chem. 2010, 2265.
[57] So, S. S.; Burkett, J. A.; Mattson, A. E. Org. Lett. 2011, 13, 716.
[58] Curti, C.; Rassu, G.; Zambrano, V.; Pinna, L.; Pelosi, G.; Sartori, A.; Battistini, L.; Zanardi, F.; Casiraghi, G. Angew. Chem., Int. Ed. 2012, 51, 1.
[59] Lin, C.; Hsu, J.; Sastry, M. N. V.; Fang, H.; Tu, Z.; Liu, J.-T.; Yao, C.-F. Tetrahedron 2005, 61, 11751.
[60] Kusurkar, R. S.; Alkobati, N. A. H.; Gokule, A. S.; Puranik, V. G. Tetrahedron 2008, 64, 1654.
[61] Chen, Y.-C.; Xie, S.-L.; Xie, Z.-F. Chin. J. Org. Chem. 2012, 32, 1970 (in Chinese).
(陈永诚, 谢绍雷, 解正峰, 有机化学, 2012, 32, 1970.)
/
| 〈 |
|
〉 |