

Chinese Journal of Organic Chemistry >
Synthesis of 6-Alkoxyl-2-propylthio-8-azapurine Nucleosides and Their Antiplatelet Aggregation Activity Evaluation
Received date: 2013-03-11
Revised date: 2013-04-04
Online published: 2013-04-17
Supported by
Project supported by the National Natural Science Foundation of China (No. 21272022).
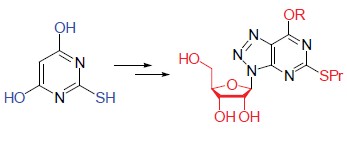
2-Thiobarbituric acid (1) was converted to 4,6-dichloro-5-nitro-2-propylthiopyrimidine (4) via S-alkylation, nitration and chlorination. Followed by azide and reduction reaction of azido, 1-O-acetyl-2,3,5-tri-O-benzoyl-β-D-ribofuranose (5) was converted to 1-amino-2,3,5-tri-O-benzoyl-β-D-ribofuranose (7). 9-[(2',3',5'-Tri-O-benzoyl)-β-D-ribofuranosyl]-6-chloro-2-propylthio-8-azapurine (10) was obtained via nucleophilic substitution of 7 with 4, reduction, diazotization and coupling reaction. Nucleophilic displacement of the chloride in 10 with various alcohols and deprotection afforded 6-alkoxyl-2-propylthio-8-azapurine nucleosides (11). Their structures were identified by 1H NMR, 13C NMR, IR and HRMS techniques. Moreover, the antiplatelet aggregation activities of compounds 11 were measured.
Deng Conger , Li Shunlai , Liu Xiangwei , Du Hongguang . Synthesis of 6-Alkoxyl-2-propylthio-8-azapurine Nucleosides and Their Antiplatelet Aggregation Activity Evaluation[J]. Chinese Journal of Organic Chemistry, 2013 , 33(08) : 1741 -1748 . DOI: 10.6023/cjoc201303017
/
| 〈 |
|
〉 |