

Chinese Journal of Organic Chemistry >
Synthesis and Spectral Properties of Water-Soluble Indocyanine Dyes as Well as the Interaction with DNA and BSA
Received date: 2012-12-24
Revised date: 2013-03-25
Online published: 2013-04-24
Supported by
Project supported by the Special Science Research Foundation of Shaanxi Education Committee (No. 11JK0558), the Scientific and Technological Research and Development Projects in Shaanxi Province (No. 2012K07-06) and the Graduate Cross-discipline Funds of Northwest University (No. 09YJC20).
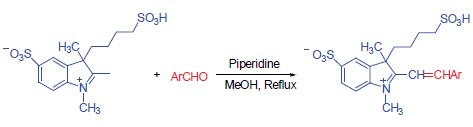
Six novel water-soluble indocyanine dyes were synthesized using 1,2,3-trimethyl-3-(4-sulphobutyl)-3H-in-dolium-5-sulphonate salt and corresponding aromatic aldehyde as materials and piperidine as catalyst. All of the compounds were characterized by 1H NMR, HRMS, IR and UV-vis techniques. Their spectral properities in different solvents were also investigated. The results showed that the maximum absorption wavelength of the dyes was in the region of 426.0~562.5 nm, the emission wavelength was located in 508.2~596.0 nm and the molar extinction coefficients was in the region of 0.6×104~6.7×104 L·mol-1·cm-1 in different solvents. The interactions of prepared dyes with salmon sperm DNA and bovine serum albumin (BSA) were also investigated. The results showed that the interaction of five water-soluble dyes with DNA or BSA was weak.
Key words: water-soluble indocyanine dye; spectral properties; DNA; BSA
Zhang Xiufu , Fu Yile , Zuo Zhijun , Hou Yuan , Li Yuexuan , Liu Yanxia , Wang Lanying . Synthesis and Spectral Properties of Water-Soluble Indocyanine Dyes as Well as the Interaction with DNA and BSA[J]. Chinese Journal of Organic Chemistry, 2013 , 33(08) : 1709 -1714 . DOI: 10.6023/cjoc201212042
/
| 〈 |
|
〉 |