

Chinese Journal of Organic Chemistry >
Claisen-Schmidt Condensation Catalyzed by Alkaline Poly(ionic liquid)
Received date: 2013-01-21
Revised date: 2013-04-03
Online published: 2013-04-24
Supported by
Project supported by the National Natural Science Foundation of China (No. 20806007) and the National Basic Research Program of China (No. 2010CB226902).
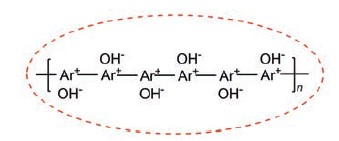
Poly(iminazole-epichlorohydri) ionic liquid was synthesized and basic poly(ionic liquid) was prepared by alkali exchange and was characterized by FT-IR, NMR, GFC, potentiometric titration methods. It turns out that the poly(ionic liquid) has perfect degree of polymerization and very concentrated relative molecular weight distribution, and the exchange ratio of hydroxyl ion can be over 95%. Claisen-Schmidt condensation reaction was efficiently catalyzed by the poly(ionic liquid). For example, taking the condesation of aldehyde and ketone (aldehyde:ketone=1:4) in 2.5 mol/L aldehyde solution with 2% (weight) catalyst at 25℃ for 10 h, the yield can reached 99%. Ultrasonic waves can enhance the reaction with a yield of 90% in 4 h. After recycling use of the catalyst for 5 times, the yield still did not less than 90%. The catalytic activity of the catalyst is higher in low addition, and catalyst could be reused well.
Song Yanlei , Wang Xincheng , Huang Chongpin , Liang Fengbing , Yu Zhichao , Chen Biaohua . Claisen-Schmidt Condensation Catalyzed by Alkaline Poly(ionic liquid)[J]. Chinese Journal of Organic Chemistry, 2013 , 33(08) : 1715 -1719 . DOI: 10.6023/cjoc201301049
/
| 〈 |
|
〉 |