

Chinese Journal of Organic Chemistry >
Synthesis and Property of Oxymethylene-Bridged Bent-core Liquid Crystals
Received date: 2013-01-16
Revised date: 2013-04-01
Online published: 2013-05-08
Supported by
Project supported by the National Natural Science Foundation of China (No.11074054), the Scientific Research Found of Hubei Provincial Education Department (No. 20091801) and the Educational and Innovative Foundation for the Graduate Students of Wuhan Polytechnic University (No. 2011cx003)
A novel series of bent-core molecules with a central bent core based on 2,5-bis(4-hydroxyphenyl)-1,3,4-oxadiazole, oxymethylene-bridged (CH2O), and alkyl tails with carbon numbers m=3, 5, 6, 7, 8, 9, 10 were prepared. Structures of the titled compounds were confirmed by 1H NMR, 13C NMR, IR, MS techniques, their transition temperatures and phase transition were investigated using differential scanning calorimetric (DSC) and polarizing optical microscopy (POM). Comparing with ester-bridged, target compounds 6a~6g have right chemical structure, possessed low melting points that below 100 ℃ (except 6c) and exhibited liquid crystal phases. Compounds 6d~6g exist wide phase temperature range and show odd-even effect.
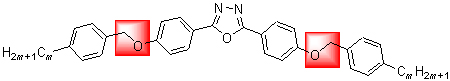
Key words: bent-core liquid crystal; oxadiazol; oxymethylene; synthesis; low melting points
Wang Yongli , Du Qiong , Wang Guohua , Zhang Zhiyong , Dai Zhiqun , Guan Jintao , Xiang Ying . Synthesis and Property of Oxymethylene-Bridged Bent-core Liquid Crystals[J]. Chinese Journal of Organic Chemistry, 2013 , 33(9) : 2010 -2015 . DOI: 10.6023/cjoc201301033
/
| 〈 |
|
〉 |