

Chinese Journal of Organic Chemistry >
Etherification of 2-Acetoxymethylpyrrole Derivative with Alcohol Catalyzed by para-Toluenesulfonic Acid
Received date: 2013-03-01
Revised date: 2013-04-28
Online published: 2013-05-08
Supported by
Project supported by the Natural Science Foundation of Jiangxi Province (No. 2009GZH0078)
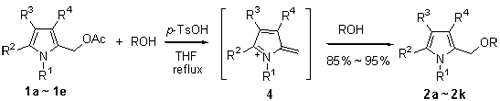
Etherification of 2-acetoxymethylpyrrole derivatives with alcohols catalyzed by para-toluenesulfonic acid was investigated in details resulting in the smooth formation of the corresponding 2-alkoxymethylpyrrole derivatives in excellent yields. An alternate method for the preparation of 2-alkoxymethylpyrrole derivatives starting from 2-acetoxymethylpyrrole derivatives under weak acidic condition was provided. The main advantages of the developed methodology are mild reaction conditions, high yields of products and simple work-up procedure. A plausible mechanism involving the generation of highly reactive azafulvene intermediate 4 was proposed to explain the observed phenomena.
Yan Zhaohua , Yu Xinquan , Liu Yongjie , Xu Yun , Lin Sen . Etherification of 2-Acetoxymethylpyrrole Derivative with Alcohol Catalyzed by para-Toluenesulfonic Acid[J]. Chinese Journal of Organic Chemistry, 2013 , 33(9) : 1975 -1981 . DOI: 10.6023/cjoc201302018
/
| 〈 |
|
〉 |