

Chinese Journal of Organic Chemistry >
Research Progress on the Synthesis of Stenine
Received date: 2013-03-31
Revised date: 2013-05-21
Online published: 2013-05-24
Supported by
Project supported by the the National Natural Science Foudation of China (Nos.21062029, 20562013, 20925205).
The alkaloid stenine which has a representative pyrrolo[1,2-a]azepine nucleus and a highly substituted perhydroindole ring system is unique among Stemona alkaloids.The polycyclic system and the seven contiguous stereogenic centers in (-)-stenine present a challenge for asymmetric organic synthesis.Progresses toward the synthesis of stenine are reviewed in terms of the key strategies employed.
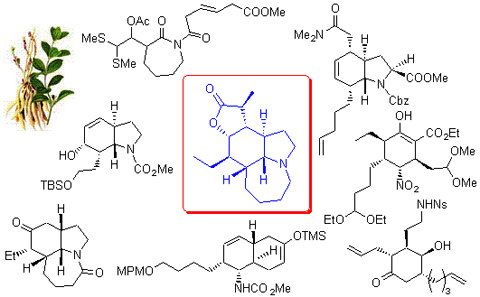
Key words: stenine; racemic synthesis; asymmitric synthesis
Chen Jingbo , Chen Jingchao , Xie Yan , Liu Nana , Liu Dandan , Zhang Hongbin . Research Progress on the Synthesis of Stenine[J]. Chinese Journal of Organic Chemistry, 2013 , 33(06) : 1186 -1194 . DOI: 10.6023/cjoc201303056
/
| 〈 |
|
〉 |