

Chinese Journal of Organic Chemistry >
Studies on the Synthesis, Crystal Structure and Antimicrobial Activities of 4-Methyl-2-heterocylic-2,3-dihydro-benzo[f]-1,5-thiazepine-3-carboxylic Acid Ethyl Ester and Its Reduction Products
Received date: 2013-05-24
Revised date: 2013-06-10
Online published: 2013-06-21
Supported by
Project supported by the National Natural Science Foundation of China (No. 20972040).
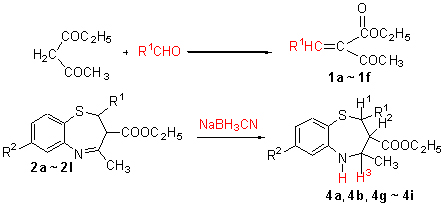
Starting from ethyl acetoacetate and substituted heterocyclic aldehyde, a series of new 4-methyl-2-heterocyclic-2,3-dihydro-benzo[f]-1,5-thiazepine-3-carboxylic acid ethyl esters (2a~2l) were synthesized through 2-step reactions, and of which five compounds were reduced. The structures of the compounds were characterized by 1H NMR, IR, MS, elemental analysis and X-ray diffraction study. The crystal of compound 2a belongs to triclinic system, space group P-1. The antibacterical activities of the new compounds against B. subtilis, C. albicans and C. neoformans were evaluated by filter paper approach, and the results indicated that most of them showed antibacterical activities, especially the compound 2a prominent.
Tian Keqing , Liu Xiaoxin , Xue Ziqiao , Yang Tian , Du Xingqiong , Zhang Ping . Studies on the Synthesis, Crystal Structure and Antimicrobial Activities of 4-Methyl-2-heterocylic-2,3-dihydro-benzo[f]-1,5-thiazepine-3-carboxylic Acid Ethyl Ester and Its Reduction Products[J]. Chinese Journal of Organic Chemistry, 2013 , 33(10) : 2237 -2243 . DOI: 10.6023/cjoc201305039
/
| 〈 |
|
〉 |