

Chinese Journal of Organic Chemistry >
A Novel Synthetic Approach of Alloursodeoxycholic Acid from Hydeoxycholic Acid
Received date: 2013-04-20
Revised date: 2013-06-25
Online published: 2013-07-03
Supported by
Project supported by the Personal Training Funds in National Basic Science of China (No. J1210040/J0104) and the Hunan Provincial Innovation Foundation for Postgraduate (No. CX2012B160).
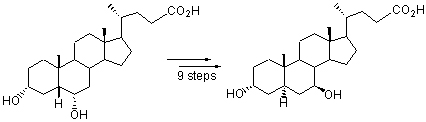
A novel and highly efficient synthetic approach of alloursodeoxycholic acid, starting from hydeoxycholic acid in 9 steps with overall yield 25%, was reported. The key intermediate 4 was prepared in good yield by improved allylic oxidation of 3. The critical steps to stereoselectively inverse the configuration of 5 and α,β-unsaturated carbonyl reduction of 6 were realized under Mistunobu coditions and Luche reduction respectively. The structures of all synthetic compounds were confirmed by 1H NMR, 13C NMR, MS and IR spectra. The synthetic approach has the advantages of easy availability of starting materials, highly stereoselective, easily work-up and good overall yield, so it has considerable practical value.
Liu Shuang , Wang Gangqiang , Liu Zhangkun , Wang Qiuan . A Novel Synthetic Approach of Alloursodeoxycholic Acid from Hydeoxycholic Acid[J]. Chinese Journal of Organic Chemistry, 2013 , 33(10) : 2216 -2219 . DOI: 10.6023/cjoc201304031
[1] Iida, T.; Momose, T.; Nambara, T.; Chang, F. C. Chem. Pharm. Bull. 1986, 34, 1929.
[2] Alvarez, M.; Jover, A.; Carrazana, J.; Meijide, F.; Soto, V. H.; Tato, J. V. Steroids 2007, 72, 535.
[3] Tohma, M.; Mahara, R.; Takeshita, H.; Kurosawa, T. Steroids 1986, 48(5~6), 331.
[4] Goto, T. Proc. Jpn. Acad. 1955, 31, 466.
[5] Shen, J. M.; Zhou, X. D.; Zhou, W. S. Acta Chim. Sinica 2006, 64, 1513 (in Chinese).(沈季铭, 周向东, 周维善, 化学学报, 2006, 64, 1513.)
[6] Iida, T.; Kakiyama, G.; Hibiya, Y.; Miyata, S.; Inoue,T.; Ohno, K.; Goto, T.; Mano, N.; Goto, J.; Nambara, T.; Hofmann, A. F. Steroids 2006, 71, 18.
[7] Wang, Z. Q.; Que, H. Q.; Jiang, L. Z.; Zhou W. S. Chin. J. Org. Chem. 1989, 9, 83 (in Chinese).(王锺麒, 阙浩泉, 姜立中, 周维善, 有机化学, 1989, 9, 83.)
[8] Ge, Y. C.; Yang, Q. X.. J. Guizhou Normal Univ. (Nat. Sci.) 2009, 27, 119 (in Chinese).(葛永昌, 杨庆雄, 贵州师范大学学报(自然科学版), 2009, 27, 119.)
[9] Xu, X. L.; Cheng, G. H., Li, X. J. Chin. J. Org. Chem. 2012, 32, 1024 (in Chinese).(许孝良, 程国华, 李小军, 有机化学, 2012, 32, 1024.)
[10] Wang, Q. A.; Luo, J. X.; Liao, T. G. Chem. J. Chin. Univ. 2004, 25, 1069 (in Chinese).(汪秋安, 罗俊霞, 廖头根, 高等学校化学学报, 2004, 25, 1069.)
[11] Gimed, A. L.; Luche, J. L. J. Am. Chem. Soc. 1981, 103, 5454
[12] Zheng, D.; Guan, Y. Y.; Chen, X. Z.; Xu, Y. P.; Chen, X. G.; Lei, P. S. Bioorg. Med. Chem. Lett. 2011, 21, 3257.
/
| 〈 |
|
〉 |