

Chinese Journal of Organic Chemistry >
Study on Synthesis of Imidazolium-Tagged Chiral Pyrphos Diphosphine Ligands and Their Applications in Asymmetric Hydrogenation
Received date: 2013-05-30
Revised date: 2013-07-06
Online published: 2013-07-10
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21172064, 20972045), the Natural Science Foundation of Hunan Province (No.10JJ2006), and the Key Scientific Research Fund of Hunan Provincial Education Department (No. 10A022).
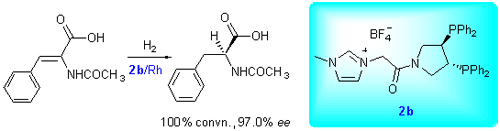
Herein we developed a facile method for preparation of a new functionalized ionic liquids supported chiral diphosphine ligands by grafting homogeneous chiral pyrphos[(R,R)-3,4-bis(diphenylphosphino)pyrrolidine] ligand with C2 axis onto imidazolium ionic liquids supports. Firstly the linker groups having halogen atoms were attached to amino group in pyrphos, and then reacted with N-methylimidazole. The other anion-containing chiral ligands located on ionic liqids (ILs) were synthesized via ion exchange technique. These ligands and the related intermediates were purified by flash column chromatography and were characterized by 1H NMR, 13C NMR, 31P NMR, IR and HRMS techniques. All results were consistent with the compounds synthesized. Then we focused on the catalytic performances and the feasibility of catalyst recycling in rhodium-catalyzed asymmetric hydrogenation of prechiral dehydroamino acid. The results showed that the catalyst generated in situ by imidazolium-tagged pyrphos ligands with BF4 as counteranion and[Rh(COD)2]BF4 exhibited high catalytic activity and enantioselectivity in asymmetric hydrogenation of α-acetamido cinnamic acid in[bmim]BF4/MeOH cosolvent systems, which was similar to the molecule catalyst. The conversion is 100%, and the ee value is up to 97.0%. Moreover, the catalyst could be reused four times without significant loss of activity and enantioselectivity.
Yi Bing , Wu Gaofeng , Zhou Wei , He Huaping . Study on Synthesis of Imidazolium-Tagged Chiral Pyrphos Diphosphine Ligands and Their Applications in Asymmetric Hydrogenation[J]. Chinese Journal of Organic Chemistry, 2013 , 33(10) : 2143 -2147 . DOI: 10.6023/cjoc201305042
/
| 〈 |
|
〉 |