

Chinese Journal of Organic Chemistry >
Amide as Nitrogen Source: Amination and Mechanism
Received date: 2013-06-04
Revised date: 2013-06-21
Online published: 2013-07-10
The mechanism of C—N bond formation with amide as nitrogen source was disclosed. The process included the cleavage of amide C—N bond to form amine and then to construct new C—N bond. With the cooperation of Lewis acid, aryl halides, aryl triflates, boronic acids, aryl siloxanes and azoles could be aminated by amide as nitrogen source.
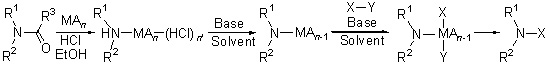
Xu Juan , Li Jiarong , Wei Zhen , Zhang Qi , Shi Daxin . Amide as Nitrogen Source: Amination and Mechanism[J]. Chinese Journal of Organic Chemistry, 2013 , 33(11) : 2435 -2439 . DOI: 10.6023/cjoc201305053
[1] (a) He,F.; Foxman,B.M.; Snider,B.B.J.Am.Chem.Soc.1998,120,6417.
(b) Wang,M.; Liang,Y.; Song,Z.G.; Jiang,H.Chin.J.Org.Chem.2010,30,295 (in Chinese).(王敏,梁燕,宋志国,姜恒,有机化学,2010,30,295.)
(c) Zhang,G.P.; Xia,Y.Chin.J.Org.Chem.2010,30,449 (in Chinese).(张国平,夏燕,有机化学,2010,30,449.)
(d) Boonen,J.; Bronselaer,A.; Nielandt,.J.; Veryser,L.; De Tre,G.; De Spiegeleer,B.J.Ethnopharmacol.2012,142,563.
(e) Wei,Y.; Shi,D.; Wei,Z.; Xu,J.; Li,J.Chin.J.Org.Chem.2012,32,1126 (in Chinese).(魏莹菲,史大昕,魏真,徐娟,李加荣,有机化学,2012,32,1126.)
[2] (a) Kim,Y.M.; Yu,S.J.Am.Chem.Soc.2003,125,1696.
(b) Zim,D.; Buchwald,S.L.Org.Lett.2003,5,2413.
(c) Ma,D.; Cai,Q.; Zhang,H.Org.Lett.2003,5,2453.
(d) Urgaonkar,S.; Nagarajan,M.; Verkade,J.G.Org.Lett.2003,5,815.
(e) Leadbeater,N.E.; Marco,M.Angew.Chem.,Int.Ed.2003,42,1407.
(f) Fang,S.; Lv,M.X.; Long,Y.H.; Yang,D.Q.Chin.J.Org.Chem.2011,31,1573 (in Chinese).(方晒,吕梅香,龙玉华,杨定乔,有机化学,2011,31,1573.)
[3] (a) Coalter,J.N.; Huffman,J.C.; Caulton,K.G.Organometallics 2000,19,3569.
(b) Zhang,M.Synthesis 2011,3408.
(c) Weibel,J.M.; Blanc,A.; Pale,P.Chem.Rev.2008,108,3149.
(d) Furuya,T.; Storm,A.E.; Ritter,T.J.Am.Chem.Soc.2009,131,1662.
[4] Bogdal,D.Molecules 1999,4,333.
[5] Demko,Z.P.; Bartsch,M.; Sharpless,K.B.Org.Lett.2000,2,2221.
[6] Xiong,T.; Li,Y.; Lv,Y.; Zhang,Q.Chem.Commun.2010,46,6831.
[7] Barros,O.S.; Nogueira,C.W.; Stangherlin,E.C.; Menezes,P.H.; Zeni,G.J.Org.Chem.2006,71,1552.
[8] (a) Zhao,J.K.; Wang,Y.G.Chin.Chem.Lett.2002,13,1149.
(b) Agarwal,A.; Chauhan,P.M.S.Synth.Commun.2004,34,2925.
(c) Cho,S.H.; Kim,J.Y.; Lee,S.Y.; Chang,S.Angew.Chem.,Int.Ed.2009,48,9127.
(d) Samadi,A.; Silva,D.; Chioua,M.; Carreiras,M.; Mar-co-Contelles,J.Synth.Commun.2011,41,2859.
(e) Chen,W.X.; Shao,L.X.J.Org.Chem.2012,77,9236.
[9] Li,J.R.; Chen,X.; Shi,D.X.; Ma,S.L.; Li,Q.; Zhang,Q.; Tang,J.H.Org.Lett.2009,11,1193.
[10] (a) Xu,J.; Shi,D.X.; Wei,Z.; Wei,Y.F.; Zhang,Q.; Li,J.R.Chin.J.Org.Chem.2012,32,776(in Chinese).(徐娟,史大昕,魏真,魏莹菲,张奇,李加荣,有机化学,2012,32,776.)
(b) Xu,J.; Li,J.R.; Wei,Z.; Zhang,Q.; Shi,D.X.RSC Adv.2013,3,9622.
[11] Xu,J.; Li,J.R.J.Chem.Res.2012,36,381.
[12] Senear,A.E.; Rapport,M.M.; Mead,J.F.J.Org.Chem.1946,11,378.
[13] Medici,A.J.A.J.Chem.Soc.,Perkin Trans.1 1977,22,2517.
[14] Kost,A.N.Zh.Obshch.Khim.1964,34,4046.
[15] Brown,B.R.J.Chem.Soc.1957,3755.
[16] Pedersen,E.B.Synthesis 1978,844.
[17] Komaromi,A.Adv.Synth.Catal.2010,352,1523.
[18] Gribble,G.W.Synthesis 1987,709.
[19] Kohler,P.C.; Ritschel,T.; Schweizer,W.B.Chem.Eur.J.2009,15,10809.
[20] Prouillac,C.; Vicendo,P.; Garrigues,J.C.Free Radicals Biol.Med.2009,46,1139.
[21] Mavrova,A.T.; Denkova,P.; Tsenov,Y.A.Biol.Med.Chem.2007,15,6291.
[22] (a) Visnjevac,A.; Tusek-Bozic,L.; Majeric-Elenkov,M.; Hamersak,Z.; Kooijman,H.; De Clercq,E.; Kojic-Prodic,B.Polyhedron 2002,21,2567.
(b) Usama,E.A.; Alaa,A.M.A.A; Shar,A.S.Eur.J.Med.Chem.2007,42,1325.
(c) Do,H.-Q.; Daugulis,O.J.Am.Chem.Soc.2009,131,17052.
(d) Bresser,T.; Mosrin,M.; Monzon,G.; Knochel,P.J.Org.Chem.2010,75,4686.
(e) Zimdars,S.; Jourdin,X.M.D.; Crestey,F.; Carell,T.; Knochel,P.Org.Lett.2011,13,792.
(f) Duez,S.; Bernhardt,S.; Heppekausen,J.; Fleming,F.F.; Knochel,P.Org.Lett.2011,13,1690.
/
| 〈 |
|
〉 |