

Chinese Journal of Organic Chemistry >
An Efficient Catalytic Synthesis and Antimicrobial Activities of Derivatives of 2-Ethoxycarbonyl(carboxyl)-4-substituted phenyl-2,3-dihydro-1H-benzo[b][1,4]diazepine
Received date: 2013-05-14
Revised date: 2013-06-07
Online published: 2013-07-11
Supported by
Project supported by the National Natural Science Foundation of China (No. 21276064), the Natural Science Foundation of Hebei Education Department (No. 2008320), and the Science Foundation of Hebei Normal University (No. 2011Y04).
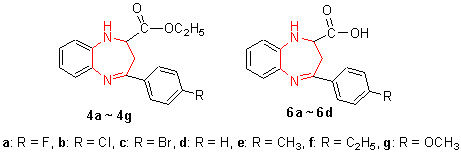
Seven novel ethoxycarboxyl-containing 2-ethoxycarbonyl-4-substituted phenyl-2, 3-dihydro-1H-benzo[b][1, 4]dia-zepines 4a~4g and four novel carboxyl-containing 2-carboxyl-4-substituted phenyl-2, 3-dihydro-1H-benzo[b]-[1, 4]diazepines 6a~6d were efficiently synthesized by the Friedel-Crafts acylation reaction, aza-Michael addition reaction, esterification, dehydration cyclization reaction, where different substituted benzene and maleic anhydride were used for the raw materials. The structures of these new compounds were determined by 1H NMR, IR, FAB mass spectral analysis and elemental analysis. The exhaustive researches on the synthetic reaction of the class of compounds were carried out and the reaction mechanism has been unequivocally established in this paper. Furthermore, newly synthesized compounds were evaluated for their antimicrobial activity. The result showed that most of the title compounds are biologically active and have obvious specificity and selective antibacterial activities to different classificatory bacterium. Density functional theory (DFT) B3LYP with 6-31G basis set has been used to investigate target compounds. Inferred N (11) and the group of C=N were active sites of inhibition bacterial (B. subtilis) and fungal (C. albicans and C. neofonmans) respectively.
Wang Lanzhi , Hua Zhongxia , Wang Shasha . An Efficient Catalytic Synthesis and Antimicrobial Activities of Derivatives of 2-Ethoxycarbonyl(carboxyl)-4-substituted phenyl-2,3-dihydro-1H-benzo[b][1,4]diazepine[J]. Chinese Journal of Organic Chemistry, 2013 , 33(11) : 2376 -2383 . DOI: 10.6023/cjoc201305022
/
| 〈 |
|
〉 |