

Chinese Journal of Organic Chemistry >
Synthesis and Crystal Structure of Azacalix[4]pyridine[4]pyrimidine
Received date: 2013-07-26
Revised date: 2013-08-23
Online published: 2013-08-29
Supported by
Project supported by the Fundamental Research Funds for the Central Universities (No. 13D110530).
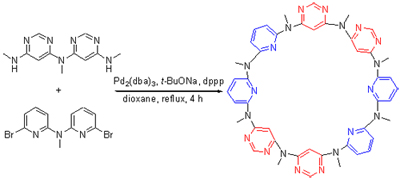
A novel azacalix[4]pyridine[4]pyrimidine was synthesized by means of a [2+2] fragment coupling approach from 2,6-dibromopyridine and 4,6-dichloropyrimidine. The lead compound was characterized by 1H NMR, 13C NMR, IR and HRMS. The crystal structure was determined by X-ray diffraction method.
Yang Zijun , Yin Chong , Zhang Linping , Zhong Yi , Xu Hong , Mao Zhiping . Synthesis and Crystal Structure of Azacalix[4]pyridine[4]pyrimidine[J]. Chinese Journal of Organic Chemistry, 2013 , 33(12) : 2607 -2611 . DOI: 10.6023/cjoc201307042
[1] (a) Lehn, J. M. Supramolecular Chemistry: Concepts and Perspectives, A Personal Account, VCH, Weinhein, 1995.
(b) Atwood, J. L.; Lehn, J. M. Comprehensive Supramolecular Chemistry, Vol. 1~10, Pregamon Press, New York, 1996.
(c) Steed, J. W.; Atwood, J. L. Supramolecular Chemistry, John Wiley & Sons, Ltd, Cambridge, UK, 2000.
[2] (a) Gutsche, C. D. Calixarenes, The Royal Society of Chemistry, Cambridge, 1989.
(b) Gutsche, C. D. Calixarenes Revisited, The Royal Society of Chemistry, Cambridge, 1998.
(c) Sameni, S.; Jeunesse, C.; Matt, D.; Harrowfield, J. Chem. Soc. Rev. 2009, 38, 2117.
(d) Nimse, S. B.; Kim, T. Chem. Soc. Rev. 2013, 42, 366.
(e) Dai, Y.-L.; Cao, Z.; Zeng, J.-L.; Gong, F.-C.; Hu, J.-L.; Hao, T.-T. Acta Chim. Sinica 2011, 69, 291 (in Chinese). (戴云林, 曹忠, 曾巨澜, 龚福春, 胡静龄, 赫婷婷, 化学学报, 2011, 69, 291.)
[3] (a) Ma, S.-L.; Guo, Q.-L.; Zhu, W.-X.; Zhao, M.-X.; Zhang, J.; Liu, Y.-C. Acta Chim. Sinica 2005, 63, 519 (in Chinese). (马淑兰, 郭倩玲, 朱文祥, 赵明新, 张静, 刘迎春, 化学学报, 2005, 63, 519.)
(b) Wang, M. X. Chem. Commun. 2008, 4541.
(c) Wang, M. X. Acc. Chem. Res. 2012, 45, 182.
(d) Xue, M.; Hu, S.-Z.; Chen, C.-F. Acta Chim. Sinica 2012, 70, 1697 (in Chinese). (薛敏, 胡树振, 陈传峰, 化学学报, 2012, 70, 1697.)
[4] (a) Xue, M.; Chen, C.-F. Org. Lett. 2009, 11, 5294.
(b) Xue, M.; Chen, C.-F. Chem. Commun. 2011, 47, 2318.
(c) Chen, C.-F. Chem. Commun. 2011, 47, 1674.
[5] König, B.; Fonseca, M. H. Eur. J. Inorg. Chem. 2000, 2303.
[6] (a) Wang, M. X.; Zhang, X. H.; Zheng, Q. Y. Angew. Chem., Int. Ed. 2004, 43, 838.
(b) Liu, S. Q.; Wang, D. X.; Zheng, Q. Y.; Wang, M. X. Chem. Commun. 2007, 3856.
[7] Wang, L. X.; Wang, D. X.; Huang, Z. T.; Wang, M. X. J. Org. Chem. 2010, 75, 741.
[8] Mathieu, S.; Gradl, S. N.; Wen, Z. Y.; Aliagas, I.; Janet, G. T.; Lee, W.; Pulk, R.; Zhao, G. L.; Bruno, A.; Boggs, J. W.; Choo, E. F.; Gould, S. E.; Georgia, H.; Ran, Y. Q.; Rudolph, J.; Ren, L.; Buckmelter, A. J.; Dinkel, V.; Gloor, S. L.; Hansen, J. D.; Hastings, G.; Laird, E. R.; Moreno, D.; Voegtli, W. C.; Wenglowsky, S.; Grina, J. J. Med. Chem. 2012, 55, 2869.
[9] (a) Zhang, E. X.; Wang, D. X.; Zheng, Q. Y.; Wang, M. X. Org. Lett. 2008, 10, 2565.
(b) Zhang, E. X.; Wang, D. X.; Huang, Z. T.; Wang, M. X. J. Org. Chem. 2009, 74, 8595.
[10] Sheldrick G. M. SHELX-97, University of Göttingen, Germany, 1997.
/
| 〈 |
|
〉 |