

Chinese Journal of Organic Chemistry >
Design, Synthesis and Characterization of Triarylacrylonitrile Compounds Exhibiting Aggregation-Induced Emission and High Contrast Reversible Mechanochromism
Received date: 2013-06-09
Revised date: 2013-08-20
Online published: 2013-09-06
Supported by
Project supported by the National Key Basic Research Program of China (973 Program, Nos. 2010CB635108, 2011CBA00700), the International Sci & Tech Cooperation Program (No. 2012DFA51210) and the National Natural Science Foundation of China (Nos. 51203138, 51273179).
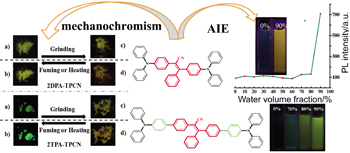
Two triarylacrylonitrile compounds, (Z)-2,3-bis(4-(diphenylamino)phenyl)-3-phenyl acrylonitrile (2DPA-TPCN) and (Z)-2,3-bis(4'-(diphenylamino)biphenyl-4-yl)-3-phenyl acrylonitrile (2TPA-TPCN) have been designed and synthesized. Characterizations were performed via nuclear magnetic resonance (NMR) and electron ionization-mass spectrometry (EI-MS). Exhibiting aggregation-induced emission (AIE) activity, the obtained 2DPA-TPCN and 2TPA-TPCN hardly have any luminescence in pure THF solution. However, strong luminescences were observed after adding water to the THF solutions (fw>60%). When 2DPA-TPCN and 2TPA-TPCN were ground, their colors and intensity turned weakly from saffron [fluorescence quantum efficiency (ΦF)=2.0%] to orange (ΦF=12.0%) and from jade green (ΦF=44.6%) to croci (ΦF=16.8%), respectively. And the change of the color of 2TPA-TPCN could be discriminated at natural light. Moreover, the ground powder returns to original colour by being exposed to solvent vapor or being heated at about 100 ℃ for 2 min. Photoluminescence (PL) spectra of the samples, which were obtained under different conditions (ungrind and grind), showed that the maximum emission wavelength (λem) had a significant red-shift as high as 8 and 39 nm after being ground, respectively. The morphological structures of 2DPA-TPCN and 2TPA-TPCN under different aggregative state were observed by scanning electron microscopy (SEM). The results indicate that the damage on the surface morphology, which was caused by external stimuli, could be restored by being exposed to ethanol vapor or heated. The X-ray diffractometry (XRD) measurements show that the dye that was not ground displays indicative of well-defined microcrystalline-like structures and the grinding dye shows amorphous features in this state. These observations further indicate that the optical properties (PL spectra, ΦF, fluorescence lifetime) of changes originate from the altering molecular packing mode from the high-order to disorder by being ground. The differential scanning calorimetry (DSC) measurements indicate that the grinding samples which have cold crystal peaks are thermodynamical metastable, while the other states are thermodynamical stable. These results further demonstrate that the primary cause of the reversibility of the grind sample might be the thermodynamical metastable. Furthermore, the thermal decomposition temperatures of 2DPA-TPCN and 2TPA-TPCN is 387.6 and 436.4 ℃ by thermogravimetry (TG) measurement, respectively, which indicates that the compounds have very good thermal stability.
Mao Wengang , Chen Kang , Ouyang Mi , Sun Jingwei , Zhou Yongbing , Wang Yongsheng , Song Qingbao , Zhang Cheng . Design, Synthesis and Characterization of Triarylacrylonitrile Compounds Exhibiting Aggregation-Induced Emission and High Contrast Reversible Mechanochromism[J]. Chinese Journal of Organic Chemistry, 2014 , 34(1) : 161 -169 . DOI: 10.6023/cjoc201306017
[1] Mutai, T.; Satou, H.; Araki, K. Nat. Mater. 2005, 4, 685.
[2] Park, S. J.; Kuang, S. G.; Fryd, M.; Saven, J. G.; Park, S. J. J. Am. Chem. Soc. 2010, 132, 9931.
[3] Dong, Y. Q.; Lam, J. W. Y.; Qin, A. J.; Liu, J. Z.; Li, Z.; Tang, B. Z. Appl. Phys. Lett. 2007, 91, 011111.
[4] Ning, Z.; Chen, Z.; Zhang, Q.; Yan, Y.; Qian, S.; Cao, Y.; Tian, H. Adv. Funct. Mater. 2007, 17, 3799.
[5] Löwe, C.; Weder, C. Adv. Mater. 2002, 14, 1625.
[6] Srinivasan, S.; Babu, P. A.; Mahesh, S.; Jayaghosh, A. A. J. Am. Chem. Soc. 2009, 131, 15122.
[7] Sagara, Y.; Kato, T. Nat. Chem. 2009, 1, 605.
[8] Chi, Z. G.; Zhang, X. Q.; Xu, B. J.; Zhou, X.; Ma, C. P.; Zhang, Y.; Liu, S. W.; Xu, J. R. Chem. Soc. Rev. 2012, 41, 3878.
[9] Song, K.; Kuzmany, H.; Wallraff, G. M.; Miller, R. D.; Rabolt, J. F. Macromolecules 1990, 23, 3870.
[10] Song, K.; Miller, R. D.; Wallraff, G. M.; Rabolt, J. F. Macromolecules 1991, 24, 4084.
[11] Song, K.; Miller, R. D.; Wallraff, G. M.; Rabolt, J. F. Macromolecules 1992, 25, 3629.
[12] Chan, H. S. O.; Ng, S. C. Prog. Polym. Sci. 1998, 23, 1167.
[13] Yamamoto, T.; Sato, T.; Iijima, T.; Abe, M.; Fukumoto, H.; Koizumi, T. A.; Usui.; M.; Nakamura, Y.; Yagi, T.; Tajima, H..; Okada, T.; Sasaki, S.; Kishida, H.; Nakamura, A.; Fukuda, T.; Emoto, A.; Ushijima, H.; Kurosaki, C.; Hirota, H. Bull. Chem. Soc. Jpn. 2009, 82, 896.
[14] Zhang, X. Q.; Chi, Z. G.; Zhang, Y.; Liu, S. W.; Xu, J. R. J. Mater. Chem. C 2013, 1, 3376.
[15] Fridman, N.; Speiser, S.; Kaftory, M. Cryst. Growth Des. 2006, 6, 1653.
[16] Gawinecki, R.; Viscardi, G.; Barni, E.; Hanna, M. A. Dyes Pigm. 1993, 23, 73.
[17] Weder, C. Nature 2009, 459, 45.
[18] Xu, B. J.; Xie, M. Y.; He, J. J.; Xu, B.; Chi, Z. G.; Tian, W. J.; Jiang, L.; Zhao, F.; Zhang, Y.; Xu, Z. Z.; Xu, J. R. Chem. Commun. 2013, 49, 273.
[19] Zhang, Z. L.; Yao, D. D.; Zhou, T. L.; Zhang, H. Y.; Wang, Y. Chem. Commun. 2011, 47, 7782.
[20] Teng, M. J.; Jia, X. R.; Yang, S.; Chen, X. F.; Wei, Y. Adv. Mater. 2012, 24, 1255.
[21] Jakubiak, R.; Collison, C. J.; Wan, W.; Rothberg, L. J.; Hsieh, B. R. J. Phys. Chem. A 1999, 103, 2394.
[22] Luo, J. D.; Xie, Z. L.; Lam, J. Y.; Cheng, L.; Chen, H. Y.; Qiu, C. F.; Kwok, H. S.; Zhan, X. W.; Liu, Y. Q.; Zhu, D. B.; Tang, B. Z. Chem. Commun. 2001, 1740.
[23] An, B. K.; Kwon, S. K.; Jung, S. D.; Park, S. Y. J. Am. Chem. Soc. 2002, 124, 14410.
[24] Luo, J. F.; Wang, X. H.; Wang, X. M.; Su, W. M. Chin. J. Chem. 2012, 30, 2488 (in Chinese).
[25] Mi, B. X.; Dong, Y. Q.; Li, Z.; Lam, J. W. Y.; Häußler, M.; Sung, H. H. Y.; Kwok, H. S.; Dong, Y. P.; Williams, I. D.; Liu, Y. Q.; Luo, Y.; Shuai, Z. G.; Zhu, D. B.; Tang, B. Z. Chem. Commun. 2005, 3583.
[26] Ning, Z.; Chen, Z.; Zhang, Q.; Yan, Y.; Qian, S.; Cao, Y.; Tian, H. Adv. Funct. Mater. 2007, 17, 3799.
[27] Li, Z.; Dong, Y. Q.; Lam, J. W. Y.; Sun, J.; Qin, A.; Häußler, M.; Dong, Y. P.; Sung, H. H. Y.; Williams, I. D.; Kwok, H. S.; Tang, B. Z. Adv. Funct. Mater. 2009, 19, 905.
[28] Toal, S. J.; Jones, K. A.; Magde, D.; Trogler, W. C. J. Am. Chem. Soc. 2005, 127, 11661.
[29] Liu, Y.; Tang, Y. H.; Barashkov, N. N.; Irgibaeva, I. S.; Lam, J. W. Y.; Hu, R. R.; Birimzhanova, D.; Yu, Y.; Tang, B. Z. J. Am. Chem. Soc. 2010, 132, 13951.
[30] Wang, M.; Zhang, G. X.; Zhang, D. Q.; Zhu, D. B.; Tang, B. Z. J. Mater. Chem. 2010, 20, 1858.
[31] Liu, Y.; Deng, C. M.; Tang, L.; Qin, A. J.; Hu, R. R.; Sun, J. Z.; Tang, B. Z. J. Am. Chem. Soc. 2011, 133, 660.
[32] Zhao, G. S.; Shi, C. X.; Guo, Z. Q.; Zhu, W. H.; Zhu, S. Q. Chin. J. Org. Chem. 2012, 32, 1620 (in Chinses).
(赵国生, 史川兴, 郭志前, 朱为宏, 朱世琴, 中国化学, 2012, 32, 1620.)
[33] Yoon, S. J.; Chung, J. W.; Gierschner, J.; Kim, K. S.; Choi, M. G.; Kim, D.; Park, S. Y. J. Am. Chem. Soc. 2010, 132, 13675.
[34] Gu, X. G.; Yao, J. J.; Zhang, G. X.; Yan, Y. L.; Zhang, C.; Peng, Q.; Liao, Q.; Wu, Y. S.; Xu, Z. Z.; Zhao, Y. S.; Fu, H. B.; Zhang, D. Q. Adv. Funct. Mater. 2012, 22, 4862.
[35] Luo, X. L.; Li, J. N.; Li, C. H.; Heng, L. P.; Dong, Y. Q.; Liu, Z. P.; Zhi, S. B.; Tang, B. Z. Adv Mater. 2011, 23, 3261.
[36] Zhou, X.; Li, H. Y.; Chi, Z. G.; Zhang, X. Q.; Zhang, Y.; Liu, S. W.; Jia, X. R. J. Fluoresc. 2012, 22, 565.
[37] Zhou, X.; Li, H. P.; Chi, Z. G.; Zhang, X. Q.; Zhang, J. Y.; Xu, B. J.; Zhang, Y.; Liu, S. W.; Jia, X. R. New J. Chem. 2012, 36, 685.
[38] Luo, X. L.; Zhao, W. J.; Shi, J. P.; Li, C. H.; Liu, Z. P.; Zhi, S. B.; Dong, Y. Q.; Tang, B. Z. J. Phys. Chem. C 2012, 116, 21967.
[39] Yoon, S. J.; Park, S. Y. J. Mater. Chem. 2011, 21, 8338.
[40] Dou, C. D.; Han, L.; Zhao, S. S.; Zhang, H. Y.; Wang, Y. J. Phys. Chem. Lett. 2011, 2, 666.
[41] Li, H.; Chi, Z. G.; Xu, B.; Zhang, X.; Li, X.; Liu, S.; Zhang, Y.; Xu, J. J. Mater. Chem. 2011, 21, 3760.
[42] Xu, B. J.; Chi, Z. G.; Zhang, X. Q.; Li, H. Y.; Chen, C. J.; Liu, S. W.; Zhang, Y.; Xu, J. R. Chem. Commun. 2011, 47, 11080.
[43] Wang, Y. L.; Wei, L.; Bu, L. Y.; Li, L. F.; Zheng, M.; Zhang, D. T.; Sun, M. X.; Tao, Y.; Xue, S. F.; Yang, W. J. J. Mater. Chem. C 2013, 1, 856.
[44] Yuan, W. Z.; Gong, Y. Y.; Chen, S. M.; Chen, X. Y.; Lam, J. W. Y.; Lu, P.; Lu, Y. W.; Wang, Z. M.; Hu, R. R.; Xie, N.; Kwok, H. S.; Zhang, Y. M.; Jing, Z. S.; Tang, B. Z. Chem. Mater. 2012, 24, 1518.
[45] Zhang, Y. J.; Sun, J. W.; Bian, G. F.; Chen, Y. Y.; Ouyang, M.; Hu, B.; Zhang, C. Photochem. Photobiol. Sci. 2012, 11, 1414.
[46] Zhang, Y. J.; Zhang, G. L.; Ouyang, M.; Hu, B.; Song, Q. B.; Sun, J. W.; Zhang, C.; Gu, C.; Xu, Y. X.; Ma, Y. G. Dyes Pigm. 2013, 98, 486.
[47] Mao, W. G.; Chen, K.; Ouyang, M.; Sun, J. W.; Zhou, Y. B.; Song, Q. B.; Zhang, C. Acta Chim. Sinica 2013, 71, 613 (in Chinese).
(毛文纲, 陈康, 欧阳密, 孙璟玮, 周永兵, 宋庆宝, 张诚, 化学学报, 2013, 71, 613.)
[48] Auweter, H.; Haberkorn, H.; Heckmann, W.; Horn, D.; Lüddecke, E.; Rieger, J.; Weiss, H. Angew. Chem., Int. Ed. 1999, 38, 2188.
[49] Jenekhe, S. A.; Osaheni, J. A. Science 1994, 265, 765.
[50] Zhang, X. Q.; Chi, Z. G.; Li, H. Y.; Xu, B. J.; Li, X. F.; Zhou, W.; Liu, S. W.; Zhang, Y.; Xu, J. R. Chem. Asian J. 2011, 6, 808.
[51] Crosby, G. A.; Demas, J. N. J. Phys. Chem. 1971, 75, 991.
/
| 〈 |
|
〉 |