

Chinese Journal of Organic Chemistry >
Progress in Transition-Metal-Catalyzed Oxidative Cross-Coupling of Terminal Alkynes
Received date: 2013-07-20
Revised date: 2013-09-03
Online published: 2013-09-17
Supported by
Project supported by the Natural Science Foundation of Shanxi Province (Nos. 2012021007-2, 2011011010-2), and the Scientific and Technological Innovation Programs of Higher Education Institutions in Shanxi Province (No. 20120006).
Alkynyl compounds are one kind of important functional compounds in organic synthesis. Various new alkynyl compounds can be obtained via oxidative cross-coupling reactions of terminal alkynes. Recent oxidative cross-coupling reactions involving terminal alkynes are reviewed. The advances of cross-coupling reactions catalyzed by Pd, Cu, Fe and so on are particularly described.
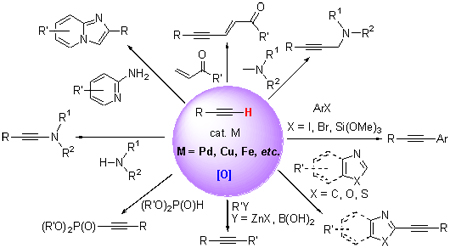
Key words: transition metal; C—H bond; terminal alkyne; oxidative cross-coupling; progress
Zhang Congxia , Li Nana , Li Xing , Chang Honghong , Liu Qiang , Wei Wenlong . Progress in Transition-Metal-Catalyzed Oxidative Cross-Coupling of Terminal Alkynes[J]. Chinese Journal of Organic Chemistry, 2014 , 34(1) : 81 -91 . DOI: 10.6023/cjoc201307024
[1] Ullmann, F. Ber. Dtsch. Chem. Ges. 1903, 36, 2382.
[2] Zhang, B. B.; Zhan, D.; Zhang, X. P.; Xiang, Q. J.; Zeng, Q. L. Acta Chim. Sinica 2012, 70, 1655 (in Chinese).
(张斌彬, 詹丹, 张小平, 向沁洁, 曾庆乐, 化学学报, 2012, 70, 1655.)
[3] Darses, S.; Genet, J. P. Chem. Rev. 2008, 108, 288.
[4] Miyaura, N.; Suzuki, A. Chem. Rev. 1995, 95, 2457.
[5] Alonso, F.; Beletskaya, I. P.; Yus, M. Tetrahedron 2005, 61, 11771.
[6] (a) Li, X.; Yan, X. Y.; Chang, H. H.; Wang, L. C.; Zhang, Y.; Chen, W. W.; Li, Y. W.; Wei, W. L. Org. Biomol. Chem. 2012, 10, 495.
(b) Li, X.; Wang, L. C.; Chang, H. H.; Zhang, C. X.; Wei, W. L. Appl. Catal. A: Gen. 2013, 462~463, 15.
[7] Wen, Y. M.; Jiang, H. F. Acta Chim. Sinica 2012, 70, 1716 (in Chinese).
(温燕梅, 江焕峰, 化学学报, 2012, 70, 1716.)
[8] Ritleng, V.; Sirlin, C.; Pfeffer, M. Chem. Rev. 2002, 102, 1731.
[9] Arockiam, P. B.; Bruneau, C.; Dixneuf, P. H. Chem. Rev. 2012, 112, 5879.
[10] Jia, C. G.; Kitamura, T.; Fujiwara, Y. Acc. Chem. Res. 2001, 34, 633.
[11] Li, H.; Li, B. J.; Shi, Z. J. Catal. Sci. Technol. 2011, 1, 191.
[12] (a) Pan, F.; Shi, Z. J. Acta Chim. Sinica 2012, 70, 1679 (in Chinese).
(潘菲, 施章杰, 化学学报, 2012, 70, 1679.;
b) Zhang, D.; Qin, Y. Acta Chim. Sinica 2013, 71, 147 (in Chinese).
(张丹, 秦勇, 化学学报, 2013, 71, 147.)
[13] Li, C. J. Acc. Chem. Res. 2009, 42, 335.
[14] Li, Z. P.; Li, C. J. J. Am. Chem. Soc. 2005, 127, 3672.
[15] Cai, G. X.; Fu, Y.; Li, Y. Z.; Wan, X. B.; Shi, Z. J. J. Am. Chem. Soc. 2007, 129, 7666.
[16] Huber, S. M.; Ertem, M. Z.; Aquilante, F.; Gagliardi, L.; Tolman, W. B.; Cramer, C. J. Chem. Eur. J. 2009, 15, 4886.
[17] Sonogashira, K. J. Organomet. Chem. 2002, 653, 46.
[18] Glaser, C. Ber. Dtsch. Chem. Ges. 1869, 2, 422.
[19] Glaser, C. Ann. Chem. Pharm. 1870, 154, 137.
[20] Kim, S. H.; Yoon, J.; Chang, S. Org. Lett. 2011, 13, 1474.
[21] Liang, B.; Dai, M. J.; Chen, J. H.; Yang, Z. J. Org. Chem. 2005, 70, 391.
[22] Li, J. H.; Liang, Y.; Xie, Y. X. J. Org. Chem. 2005, 70, 4393.
[23] Hadi, V.; Yoo, K. S.; Jeong, M.; Jung, K. W. Tetrahedron Lett. 2009, 50, 2370.
[24] Chen, M.; Zheng, X. L.; Li, W. Q.; He, J.; Lei, A. W. J. Am. Chem. Soc. 2010, 132, 4101.
[25] Luh, T. Y.; Leung, M. K.; Wong, K. T. Chem. Rev. 2000, 100, 3187.
[26] Facoetti, D.; Abbiati, G.; d'Avolio, L.; Ackermann, L.; Rossi, E. Synlett 2009, 2273.
[27] Yue, D. W.; Larock, R. C. Org. Lett. 2004, 6, 1037.
[28] Zhang, H. M.; Larock, R. C. J. Org. Chem. 2002, 67, 7048.
[29] Gu, Y. H.; Wang, X. M. Tetrahedron Lett. 2009, 50, 763.
[30] Besselièvre, F.; Piguel, S. Angew. Chem., Int. Ed. 2009, 48, 9553.
[31] Brand, J. P.; Charpentier, J.; Waser, J. Angew. Chem., Int. Ed. 2009, 48, 9346.
[32] Yang, L.; Zhao, L.; Li, C. J. Chem. Commun. 2010, 46, 4184.
[33] Zou, G.; Zhu, J. R.; Tang, J. Tetrahedron Lett. 2003, 44, 8709.
[34] Yang, F.; Wu, Y. J. Eur. J. Org. Chem. 2007, 3476.
[35] Ye, Z. S.; Liu, M. C.; Lin, B. D.; Wu, H. Y.; Ding, J. C.; Cheng, J. Tetrahedron Lett. 2009, 50, 530.
[36] Wu, C. R.; Li, P.; Fang, Y. S.; Zhao, J. J.; Xue, W. C.; Li, Y.; Larock, R. C.; Shi, F. Tetrahedron Lett. 2011, 52, 3797.
[37] Jie, X. M.; Shang, Y. P.; Hu, P.; Su, W. P. Angew. Chem., Int. Ed. 2013, 52, 3535.
[38] Zhou, L.; Ye, F.; Ma, J. C.; Zhang, Y.; Wang, J. B. Angew. Chem., Int. Ed. 2011, 50, 3510.
[39] Hamada, T.; Ye, X.; Stahl, S. S. J. Am. Chem. Soc. 2008, 130, 833.
[40] Wang, L.; Huang, H.; Priebbenow, D. L.; Pan, F. F.; Bolm, C. Angew. Chem., Int. Ed. 2013, 52, 3478.
[41] Wei, Y.; Zhao, H. Q.; Kan, J.; Su, W. P.; Hong, M. C. J. Am. Chem. Soc. 2010, 132, 2522.
[42] Matsuyama, N.; Kitahara, M.; Hirano, K.; Satoh, T.; Miura, M. Org. Lett. 2010, 12, 2358.
[43] Iorga, B.; Eymery, F.; Carmichael, D.; Savignac, P. Eur. J. Org. Chem. 2000, 3103.
[44] Lera, M.; Hayes, C. J. Org. Lett. 2000, 2, 3873.
[45] Gao, Y. X.; Wang, G.; Chen, L.; Xu, P. X.; Zhao, Y. F.; Zhou,Y. B.; Han, L. B. J. Am. Chem. Soc. 2009, 131, 7956.
[46] Kitahara, M.; Hirano, K.; Tsurugi, H.; Satoh, T.; Miura, M. Chem. Eur. J. 2010, 16, 1772.
[47] Chu, L. L.; Qing, F. L. J. Am. Chem. Soc. 2010, 132, 7262.
[48] Li, Z. P.; Li, C. J. J. Am. Chem. Soc. 2004, 126, 11810.
[49] Zhao, L.; Li, C. J. Angew. Chem., Int. Ed. 2008, 47, 7075.
[50] Bi, H. P.; Zhao, L.; Liang, Y. M.; Li, C. J. Angew. Chem., Int. Ed. 2009, 48, 792.
[51] Niu, M. Y.; Yin, Z. M.; Fu, H.; Jiang, Y. Y.; Zhao, Y. F. J. Org. Chem. 2008, 73, 3961.
[52] Pan, C. D.; Luo, F.; Wang, W. H.; Ye, Z. S.; Cheng, J. Tetrahedron Lett. 2009, 50, 5044.
[53] Jiang, X. L.; Chu, L. L.; Qing, F. L. Org. Lett. 2012, 14, 2870.
[54] Xu, X. L.; Li, X. N. Org. Lett. 2009, 11, 1027.
[55] Xu, Z. W.; Yu, X. Q.; Feng, X. J.; Bao, M. J. Org. Chem. 2011, 76, 6901.
[56] Stavropoulos, P.; Çelenligil-Çetin, R.; Tapper, A. E. Acc. Chem. Res. 2001, 34, 745.
[57] Walling, C. Acc. Chem. Res. 1998, 31, 155.
[58] Volla, C. M. R.; Vogel, P. Org. Lett. 2009, 11, 1701.
[59] (a) Horner, L.; Junkermann, H. Ann. Chem. Justus Liebig 1955, 591, 53.
(b) Horner, L.; Kirmse, W. Ann. Chem. Justus Liebig 1955, 597, 48.
[60] Meng, X.; Li, C. B.; Han, B. C.; Wang, T. S.; Chen, B. H. Tetrahedron 2010, 66, 4029.
[61] You, X. L.; Xu, L.; Hu, T. Lett. Org. Chem. 2012, 9, 300.
[62] Yin, W. Y.; He, C.; Chen, M.; Zhang, H.; Lei, A. W. Org. Lett. 2009, 11, 709.
[63] He, C.; Hao, J.; Xu, H.; Mo, Y. P.; Liu, H. Y.; Han, J. J.; Lei, A. W. Chem. Commun. 2012, 48, 11073.
[64] He, C.; Guo, S.; Ke, J.; Hao, J.; Xu, H.; Chen, H. Y.; Lei, A. W. J. Am. Chem. Soc. 2012, 134, 5766.
[65] de Haro, T.; Nevado, C. J. Am. Chem. Soc. 2010, 132, 1512.
/
| 〈 |
|
〉 |