

Chinese Journal of Organic Chemistry >
Progress of Nickel-Catalyzed Suzuki-Miyaura Cross-Coupling Reactions of Phenol Derivatives
Received date: 2013-07-23
Revised date: 2013-09-06
Online published: 2013-09-25
Suzuki-Miyaura coupling reaction catalyzed by transition-metal nickel is widely used in organic synthesis. In this review, the development of transition-metal nickel-catalyzed cross-coupling reactions is summarized, mainly including aryl sulfonates, aryl sulfamates, aryl carbonates, aryl carboxylates, aryl carbamates, aryl phosphorus derivatives, aryl ethers, aryl heteroaryl ethers and phenolate.
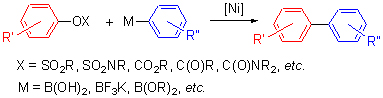
Chen Guojun , Du Jianshi . Progress of Nickel-Catalyzed Suzuki-Miyaura Cross-Coupling Reactions of Phenol Derivatives[J]. Chinese Journal of Organic Chemistry, 2014 , 34(1) : 65 -80 . DOI: 10.6023/cjoc201307035
[1] Zhang, D.; Qin, Y. Acta Chim. Sinica 2013, 71, 147 (in Chinese).
(张丹, 秦勇, 化学学报, 2013, 71, 147.)
[2] Wen, Y.-M.; Jiang, H.-F. Acta Chim. Sinica 2012, 70, 1716 (in Chinese).
(温燕梅, 江焕峰, 化学学报, 2012, 70, 1716.)
[3] Pan, F.; Shi, Z.-J. Acta Chim. Sinica 2012, 70, 1679 (in Chinese).
(潘菲, 施章杰, 化学学报, 2012, 70, 1679.)
[4] Jin, L.-Q.; Luo, X.-C.; Lei, A.-W. Acta Chim. Sinica 2012, 70, 1538 (in Chinese).
(靳立群, 罗贤才, 雷爱文, 化学学报, 2012, 70, 1538.)
[5] Hassan, J.; Sevignom, M.; Gozzi, C.; Schulz, E.; Lemaire, M. Chem. Rev. 2002, 102, 1359.
[6] Rosen, B. M.; Quasdorf, K. W.; Wilson, D. A.; Zhang, N.; Resmerita, A.-M.; Garg, N. K.; Percec, V. Chem. Rev. 2011, 111, 1346.
[7] Miyaura, N.; Suzuki, A. Chem. Rev. 1995, 95, 2457.
[8] Jana, R.; Pathak, T. P.; Sigman, M. S. Chem. Rev. 2011, 111, 1417.
[9] Han, F.-S. Chem. Soc. Rev. 2013, 42, 5270.
[10] Percec, V.; Bae, J.-Y.; Hill, D. H. J. Org. Chem. 1995, 60, 1060.
[11] Ueda, M.; Saitoh, A.; Oh-tani, S.; Miyaura, N. Tetrahedron 1998, 54, 13079.
[12] Zim, D.; Lando, V. R.; Dupont, J.; Monteiro, A. L. Org. Lett. 2001, 3, 3049.
[13] Kobayashi, Y.; Mizojiri, R. Tetrahedron Lett. 1996, 37, 8531.
[14] Kobayashi, Y.; William, A. D.; Mizojiri, R. J. Organomet. Chem. 2002, 653, 91.
[15] Tang, Z.-Y.; Hu, Q.-S. J. Am. Chem. Soc. 2004, 126 , 3058.
[16] Tang, Z.-Y.; Spinella, S.; Hu, Q.-S. Tetrahedron Lett. 2006, 47, 2427.
[17] Wilson, D. A.; Wilson, C. J.; Rosen, B. M.; Percec, V. Org. Lett. 2008, 10, 4879.
[18] Leowanawat, P.; Zhang, N.; Resmerita, A.-M.; Rosen, B. M.; Percec, V. J. Org. Chem. 2011, 76, 9946.
[19] Lipshutz, B. H.; Butler, T.; Swift, E. Org. Lett. 2008, 10, 697.
[20] Kuroda, J.; Inamoto, K.; Hiroya, K.; Doi, T. Eur. J. Org. Chem. 2009, 2251.
[21] Tu, T.; Mao, H.; Herbert, C.; Xu, M.-Z.; Dötz, K. H. Chem. Commun. 2010, 46, 7796.
[22] Molander, G. A.; Beaumard, F. Org. Lett. 2010, 12, 4022.
[23] Fan, X.-H.; Yang, L.-M. Eur. J. Org. Chem. 2010, 2457.
[24] Fan, X.-H.; Yang, L.-M. Eur. J. Org. Chem. 2011, 1467.
[25] Leowanawat, P.; Zhang, N.; Safi, M.; Hoffman, D. J.; Fryberger, M. C.; George, A.; Percec, V. J. Org. Chem. 2012, 77, 2885.
[26] Xing, C.-H.; Lee, J.-R.; Tang, Z.-Y.; Zheng, J.-R.; Hu, Q.-S. Adv. Synth. Catal. 2011, 353, 2051.
[27] Leowanawat, P.; Zhang, N.; Percec, V. J. Org. Chem. 2012, 77, 1018.
[28] Zhang, N.; Hoffman, D. J.; Gutsche, N.; Gupta, J.; Percec, V. J. Org. Chem. 2012, 77, 5956.
[29] Gao, H.; Li, Y.; Zhou, Y.-G.; Han, F.-S.; Lin, Y.-J. Adv. Synth. Catal. 2011, 353, 309.
[30] Quasdorf, K. W.; Riener, M.; Petrova, K. V.; Garg, N. K. J. Am. Chem. Soc. 2009, 131, 17748.
[31] Quasdorf, K. W.; Antoft-Finch, A.; Liu, P.; Silberstein, A. L.; Komaromi, A.; Blackburn, T.; Ramgren, S. D.; Houk, K. N.; Snieckus, V.; Garg, N. K. J. Am. Chem. Soc. 2011, 133, 6352.
[32] Baghbanzadeh, M.; Pilger, C.; Kappe, C. O. J. Org. Chem. 2011, 76, 1507.
[33] Chen, G.-J.; Han, F.-S. Eur. J. Org. Chem. 2012, 3575.
[34] Kuwano, R.; Shimizu, R. Chem. Lett. 2011, 40, 913.
[35] Guan, B.-T.; Wang, Y.; Li, B.-J.; Yu, D.-G.; Shi, Z.-J. J. Am. Chem. Soc. 2008, 130, 14468.
[36] Quasdorf, K. W.; Tian, X.; Garg, N. K. J. Am. Chem. Soc. 2008, 130, 14422.
[37] Li, Z.; Zhang, S.-L.; Fu, Y.; Guo, Q.-X.; Liu, L. J. Am. Chem. Soc. 2009, 131, 8815.
[38] Antoft-Finch, A.; Blackburn, T.; Snieckus, V. J. Am. Chem. Soc. 2009, 131, 17750.
[39] Xu, L.; Li, B.-J.; Wu, Z.-H.; Lu, X.-Y.; Guan, B.-T.; Wang, B.-Q.; Zhao, K.-Q.; Shi, Z.-J. Org. Lett. 2010, 12, 884.
[40] Zhao, Y.-L.; Li, Y.; Li, Y.; Gao, L.-X.; Han, F.-S. Chem.-Eur. J. 2010, 16, 4991.
[41] Chen, G.-J.; Huang, J.; Gao, L.-X.; Han, F.-S. Chem.-Eur. J. 2011, 17, 4038.
[42] Li, S.-M.; Huang, J.; Chen, G.-J.; Han, F.-S. Chem. Commun. 2011, 47, 12840.
[43] Chen, H.; Huang, Z.-B.; Hu, X.-M.; Tang, G.; Xu, P.-X.; Zhao, Y.-F.; Cheng, C.-H. J. Org. Chem. 2011, 76, 2338.
[44] Tobisu, M.; Shimasaki, T.; Chatani, N. Angew. Chem., Int. Ed. 2008, 47, 4866.
[45] Li, X.-J.; Zhang, J.-L.; Geng, Y.; Jin, Z. J. Org. Chem. 2013, 78, 5078.
[46] Yu, D.-G.; Shi, Z.-J. Angew. Chem., Int. Ed. 2011, 50, 7097.
/
| 〈 |
|
〉 |