

Chinese Journal of Organic Chemistry >
Formal Synthesis of (-)-Frontalin
Received date: 2013-08-05
Revised date: 2013-09-21
Online published: 2013-09-25
Supported by
Project supported by the National Natural Science Foundation of China (No. 21372205) and the Graduate Research Project of Zhengzhou University (No. 2Y03303).
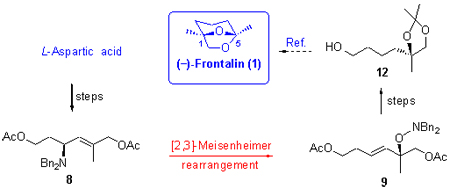
(-)-Frontalin is usually used as one of standard molecules to test the usefulness of chiral tertiary alcohol construction. Based on methodology of chiral tertiary alcohols construction via [2,3]-Meisenheimer rearrangement which has been developing on our laboratory, the formal synthesis of (-)-frontalin is finished via successive Wittig reaction, ester reduction, [2,3]-Meisenheimer rearrangement, and etc. The total yield is 34% and the ee value is 94%. The substrate scope is expanded from α,β-conjugated olefins into isolated olefins. This proves the more general characteristic of chiral tertiary alcohol construction via [2,3]-Meisenheimer rearrangement.
Zhou Hang , Sun Moran , Cao Qiwei , Zhu Ming , Bai Leiyang , Xie Yangla , Yang Hua . Formal Synthesis of (-)-Frontalin[J]. Chinese Journal of Organic Chemistry, 2013 , 33(12) : 2515 -2519 . DOI: 10.6023/cjoc201308005
[1] (a) Singh, S.; Guiry, P. J. Tetrahedron 2010, 66, 5701. For previous enantioselective synthesis, see references cited therein.
(b) Mori, K. Biosci. Biotechnol. Biochem. 2011, 75, 976.
[2] Perrin, T. E.; Rasmussen, L. E. L.; Gunawardena, R.; Rasmussen, R. A. J. Chem. Ecol. 1996, 22, 207.
[3] Greenwood, D. R.; Comeskey, D.; Hunt, M. B.; Rasmuseen, L. E. L. Nature 2005, 438, 1097.
[4] Stymiest, J. L.; Bagutski, V.; French, R. M.; Aggarwal, V. K. Nature 2008, 456, 778.
[5] Yang, H.; Sun, M.; Zhao, S.; Zhu, M.; Xie, Y.; Niu, C.; Li, C. J. Org. Chem. 2013, 78, 339.
[6] (a) Sun, M.; Xie, Y.; Gu, J.; Yang, H. Can. J. Chem. 2013, 91, 738.
(b) Sun, M.; Gao, K.; Zheng, J.; Lai, Y.; Yang, H. Res. Chem. Intermed., currently published online, DOI 10.1007/s11164-013-1263-4.
[7] Fujii, T.; Itaya, T.; Matsubara, S. Chem. Pharm. Bull. 1989, 37, 1758.
[8] Henegar, K. E.; Ashford, S. W.; Baughman, T. A.; Sih, J. C.; Gu, R.-L. J. Org. Chem. 1997, 62, 6588.
[9] Davies, S. G.; Smyth, G. D. J. Chem. Soc., Perkin Trans. 1 1996, 2467.
[10] (a) Guarna, A.; Occhiato, E. G.; Pizzetti, M.; Scarpi, D.; Sisi, S.; Sterkenburg, M. Tetrahedron: Asymmetry 2000, 11, 4227.
(b) Davies, S. G.; Smyth, G. D. Tetrahedron: Asymmetry 1996, 7, 1001.
[11] Hoffmann, R. W. Chem. Rev. 1989, 89, 1841.
[12] Ortiz, B.; Sánchez-Obregón, R.; Toscano, R.; Yuste, F. Synthesis 2008, 2105.
/
| 〈 |
|
〉 |