

Chinese Journal of Organic Chemistry >
Synthesis and Biological Activity of O-Alkyl α-(Substituted phenoxyacetoxy)alkylphosphonate
Received date: 2013-08-12
Revised date: 2013-09-06
Online published: 2013-09-25
Supported by
Project supported by the National Basic Research Program of China (973 Program, No. 2010CB126100), the National Natural Science Foundation of China (No. 21172090) and the Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT, No. IRT0953).
For the purpose of studying the structure-activity relationship of O-alkyl α-(substituted phenoxyacetoxy)alkylphosphonic derivatives, fourteen novel O-alkyl α-(substituted phenoxyacetoxy)alkylphosphonates were synthesized by the reactions of key intermediate 3 with NaI or LiBr. The intermediate 3 was prepared by the treatment of α-hydroxyalkylphosphonates 1 with substituted phenoxyacetyl chlorides 2 which could be easily obtained by the reaction of substituted phenoxyacetic acids with excess thionyl chloride. The structures of compounds 4 have been confirmed by 1H NMR, IR, MS and elemental analyses. The results of preliminary bioassay indicated that most of title compounds possess significant herbicidal and fungicidal activities. Compound 4a showed 100% inhibitory activity against all the tested species in the rate of 1.5 kg/ha. Compounds 4h~4m exhibited more than 90% inhibitory activity against Sclerotinia sclerotiorum and Botrytis cinerea in the rate of 50 μg/g.
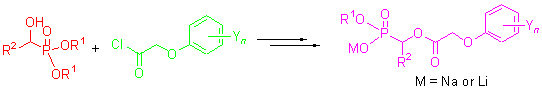
Key words: synthesis; phosphonate; herbicidal activity; fungicidal activity
Wang Tao , Zou Peng , Peng Hao , He Hongwu . Synthesis and Biological Activity of O-Alkyl α-(Substituted phenoxyacetoxy)alkylphosphonate[J]. Chinese Journal of Organic Chemistry, 2014 , 34(1) : 215 -219 . DOI: 10.6023/cjoc201308014
[1] Baillie, A. C.; Wright, K.; Wright, B. J.; Earnshaw, C. G. Pestic. Biochem. Physiol. 1988, 30, 103.
[2] Kluger, R.; Pike, D. C. J. Am. Chem. Soc. 1977, 99, 4504.
[3] Kluger, R.; Gish, G.; Kauffman, G. J. Biol. Chem. 1984, 259, 8960.
[4] He, H. W.; Yuan, J. L. Peng, H.; Chen, T.; Shen, P.; Wan, S. Q.; Li, Y. J.; Tan, H. L.; He, Y. H.; He, J. B.; Li, Y. J. Agric. Food Chem. 2011, 59, 4801.
[5] Wang, T.; He, H. W.; Miao, F. M. Chin. J. Org. Chem. 2009, 29, 1152 (in Chinese).
(贺红武, 刘建超, 缪方明, 有机化学, 2009, 29, 1152.)
[6] He, H. W.; Wang, T.; Yuan, J. L. J. Organomet. Chem. 2005, 690, 2608.
[7] Peng, H.; Wang, T.; Xie, P.; Chen, T.; He, H. W.; Wang, J. J. Agric. Food Chem. 2007, 55, 1871.
[8] Wang, T.; He, H. W. Synth. Commun. 2004, 34, 1415.
[9] He, H. W.; Peng, H.; Wang, T.; et al.Wang, C. B.; Yuan, J. L.; Chen, T.; He, J. B.; Tan, X. S., J. Agric. Food Chem. 2013, 61, 2479.
[10] Texier-Boullet, F.; Foucaud, A. Synthesis 1982, 916.
[11] Peng, H.; He, H. W. Chin. J. Org. Chem. 2007, 27, 502 (in Chinese).
(彭浩, 贺红武, 有机化学, 2007, 27, 502.)
[12] Liu, J. C.; Cui, Z. P.; He, H. W. Chin. J. Org. Chem. 2011, 31, 2082 (in Chinese).
(刘建超, 崔泽平, 贺红武, 有机化学, 2011, 31, 2082.)
[13] .Lei, J. P.; Han, J. T.; X, Z. H.; Dong, H. B.; Wang, M. A. Chin. J. Org. Chem. 2012, 32, 1993 (in Chinese).
(雷建平, 韩金涛, 徐志红, 董宏波, 王明安, 有机化学, 2012, 32, 1993.)
/
| 〈 |
|
〉 |