

Chinese Journal of Organic Chemistry >
Synthesis, Alkylation, Reduction and Acylation of Halo-functionalized Isatins
Received date: 2013-07-14
Revised date: 2013-09-14
Online published: 2014-09-30
Supported by
Project supported by the Natural Science Foundation of Liaoning Province (No. 20120201).
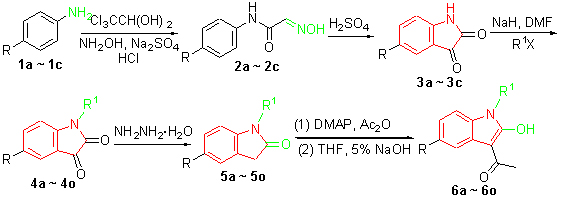
In this paper, 4-haloanilines were first underwent the Sandmeyer reaction to give the corresponding halo-functionalized isatins 3a~3c, which were further alkylated at the nitrogen atom to give 4a~4o followed by in situ reduction using hydrazine hydrate to obtain the oxindole products 5a~5o. 5a~5o were subjected to the acetylation reaction by the treatment with acetic anhydride in the presence of a catalytic amount of N,N-dimethylaminopyridine to afford the intermediates of 2-acetoxy-3-acetylindoles, which were used in next step without further purifucation. Subsequently, the hydrolysis reaction of the arising ester functions of the intermediates was carried out in tetrahydrofuran (THF) with the presence of 5% aq. NaOH at room temperature to give the targeted compounds 6a~6o. Some compounds synthesized are novel. Their structures were confirmed by 1H NMR, 13C NMR, IR, MS and elemental analysis.
Key words: halo-functionalized isatin; reduction; alkylation; acetylation
Gao Wentao , Zhao Pengbo , Zhao Binbin , Li Yang . Synthesis, Alkylation, Reduction and Acylation of Halo-functionalized Isatins[J]. Chinese Journal of Organic Chemistry, 2014 , 34(1) : 126 -136 . DOI: 10.6023/cjoc201307020
[1] Jun, Q. R.; Ning, H.; Hui, X.; Liu, M. Y.; Min, L.; Yong, T. Z. Bioorg. Med. Chem. Lett. 2010, 20, 3534.
[2] Pais, G. C. G.; Zhang, X.; Marchand, C.; Neamati, N.; Cowansage, K.; Svarovskaia, E. S.; Pathak, V. K.; Tang, Y.; Nicklaus, M.; Pommier, Y.; Burke, Jr. T. R. J. Med. Chem. 2002, 45, 3184.
[3] Ferro, S.; Barreca, M. L.; Luca, L. D.; Rao, A.; Monforte, A. M.; Debyser, Z.; Witrouw, M.; Chimirri, A. Arch. Pharm. Chem. Life Sci. 2007, 340, 292.
[4] Abdel-gawad, H.; Mohamed, H. A.; Dawood, K. M.; Badria, F. A.-R. Chem. Pharm. Bull. 2010, 58, 1529.
[5] Sriniva, P.; Raghavan, S. A. V.; Jagadeeh-bababu, R.; Gupta, C. N. V. H. B.; Sridhar, N.; Veeranjaneyulu, A.; Parimoo, P. Pharmacol. Commun. 1999, 5, 95.
[6] Giraud, F.; Alves, G.; Debiton, E.; Nauton, L.; Thery, V.; Durieu, E.; Ferandin, Y.; Lozach, O.; Meijer, L.; Anizon, F.; Pereira, E.; Moreau, P. J. Med. Chem. 2011, 54, 4474.
[7] Chennamaneni, S.; Zhong, B.; Lama, R.; Su, B. Eur. J. Med. Chem. 2012, 56, 17.
[8] Bruel, A.; Logé, C.; Tauzia, M. L.; Ravache, M.; Guevel, R.; Guillouzo, C.; Lohier, J. F.; Oliveira, Santos. J. S.; Lozach, O.; Meijer, L.; Ruchaud, S.; Bénédetti, H.; Robert, J. M. Eur. J. Med. Chem. 2012, 57, 225.
[9] Martel-Frachet, V.; Kadri, M.; Boumendjel, A.; Ronot, X. Bioorg. Med. Chem. 2011, 19, 6143.
[10] Kumar, D.; Kumar, N. M.; Sundaree, S.; Johnson, E. O.; Shah, K. Eur. J. Med. Chem. 2010, 45, 1244.
[11] Subba Reddy, B. V. S.; Rajeswari, N.; Sarangapani, M.; Reddy, G. R.; Msdan, C.; Kumar, K. P.; Rao, M. S. Bioorg. Med. Chem. Lett. 2011, 21, 6510.
[12] Subba, R. B. V.; Rajeswari, N.; Sarangapani, M.; Prashanthi, Y.; Ganji, R. J.; Addlagatta, A. Bioorg. Med. Chem. Lett. 2012, 22, 2460.
[13] Jørgensen, M.; Jørgensen, P. N.; Christoffersen, C. T.; Jensen, K. G.; Balle, T.; Bang-Andersen, B. Bioorg. Med. Chem. 2013, 21, 196.
[14] Shen, X. Q.; Wu Y. L.; Qian, H. J. Chem. Ind. Times 2012, 26, 29 (in Chinese).
(沈学全, 吴金龙, 钱海均, 化工时刊, 2012, 26, 29.)
[15] Zhang, X. F.; Liu, H. Y.; Gao, W. T. J. Bohai Univ. (Nat. Sci.) 2009, 30, 212 (in Chinese).
(张晓飞, 刘华业, 高文涛, 渤海大学学报(自然科学版), 2009, 30, 212.)
[16] Singh, R. P.; Majumder, U.; Shreeve, J. M. J. Org. Chem. 2001, 19, 6263.
[17] Lei, J.; Fang, Q.; Yuan, M.-S.; Liu, Z.-Q.; Shen, Y.-X.; Chen, H.-F. Org. Lett. 2010, 22, 5192.
[18] Hennessy, E. J.; Buchwald, S. L. J. Am. Chem. Soc. 2003, 40, 12084.
[19] Prandi, C.; Occhiato, E. G.; Tabasso, S.; Bonfante, P.; Scarpi, D.; Bova, M. E.; Miletto, I. Eur. J. Org. Chem. 2011, 20, 3781.
[20] Hamaue, N.; Mimami, M.; Terado, M.; Hirafuji, M.; Endo, T.; Machida, M.; Hiroshige, T.; Ogata, A.; Tashiro, K.; Saito, H.; Parrez, S. Neurotoxicology 2004. 25, 205.
[21] Igosheva, N.; Lorz, C.; Conner, E. Neurochem. Int. 2005, 47, 216.
[22] Minami, M.; Hamaue, N.; Hirafuji, M.; Saito, H.; Hiroshige, T.; Ogata, A.; Tashiro, K.; Parvez, S. H. J. Neural Transm. Suppl. 2006, 71, 87.
[23] Aulabaugh, A.; Kapoor, B.; Huang, X. Y.; Dollings, P.; Hum, W. T.; Banker, A.; Wood, A.; Ellestad, G. Biochemistry 2007, 46, 9462.
[24] Ogata, A.; Hamaue, N.; Terado, M.; Mimami, M.; Nagashima, K.; Tashiro, K. J. Neurol. Sci. 2003, 206, 79.
[25] Kenner, C.; Rice, B. J.; Boone, A. B.; Rubin, T. J. J. Med. Chem. 1976, 19, 887.
[26] Welstead, Jr.; W. J.; Moran, H. W.; Stauffer, H. F.; Turnbull, L. B.; Sancilio, L. F. J. Med. Chem. 1979, 22, 1074.
[27] Rivalle, C.; Bisagni, E. J. Heterocycl. Chem. 1997, 34, 441.
[28] Gassman, P. G.; Cue Jr. B. W.; Luh, T. Y. J. Org. Chem. 1977, 42, 1344.
[29] Lackey, K.; Besterman, J. M.; Fletcher, W.; Leitner, P.; Morton, B.; Sternbach, D. D. J. Med. Chem. 1995, 38, 906
[30] Garden, S. J.; Torees, J.; Ferrira, A. A.; Silva, R. B.; Pinto, A. C. Tetrahedron Lett. 1997, 38, 1501.
[31] Kawaguchi, H.; Mizuta, Y.; Sugai, F.; Saito, S. EP 19950308606, 1996 [Chem. Abstr. 1996, 125, 181167].
[32] Friedman, S. J. US 3659011, 1972 [Chem. Abstr. 1972, 77, 125053].
[33] Marti, C.; Carreira, E. M. J. Am. Chem. Soc. 127, 11505.
[34] Cui, X. J.; Shi, F.; Zhang, Y. Tetrahedron Lett. 2010, 51, 2048.
[35] Matos, I.; Prez-Mayora, E.; Soriano, E.; Zukal, A.; Martín-Aranda R. M.; López-Peinado, A. J.; Fonseca, I.; ?ejka, J. Chem. Eng. J. 2010, 161, 377.
[36] Reddy, C. R.; Jithender, E. Tetrahedron Lett. 2009, 50, 5633.
[37] Shah, H. C.; Shah, V. H.; Desai, N. D. ChemInform 2010, 40, 540.
[38] Jha, M.; Chou, T. Y.; Blunt, B. Tetrahedron 2011, 67, 982.
/
| 〈 |
|
〉 |