

Chinese Journal of Organic Chemistry >
Synthesis and Cdc25B Inhibition Activity of Novel Imidazo[2,1-b][1,3,4]thiadiazole Derivatives
Received date: 2013-09-06
Revised date: 2013-10-28
Online published: 2013-11-05
Supported by
Project supported by the Natural Science Foundation of Liaoning Province (No. 20102126).
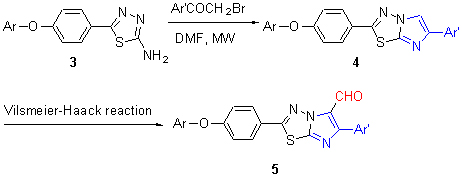
Researches indicate that the cell division cycle 25 phosphatase B (Cdc25B) is over-expressed in various primary human cancers including breast, colon, cervix, lung, etc. This suggests that the inhibition of Cdc25B may become a promising strategy in oncology. In this work, twenty novel 2,6-diaryl-imidazo[2,1-b][1,3,4]thiadiazoles 4 have been synthesized by microwave-assisted method. Then nineteen novel 2,6-diaryl-imidazo[2,1-b][1,3,4]thiadiazole-5-carbaldehydes (5) have obtained by Vilsmeier-Haack reaction. The structures of new compounds 3, 4 and 5 were characterized by IR, 1H NMR spectra and elemental analysis. The synthesized target compounds 4 and 5 were screened for Cdc25B inhibitory activities. The results revealed that compound 4c showed the highest inhibitory activity against Cdc25B with percentage inhibition 87.68% at 5 μg/mL, compounds 4o and 5c showed moderate activities with percentage inhibition 55.76% and 57.69%, respectively. They can be considered as potential candidates as novel Cdc25B inhibitors.
Key words: imidazo[2,1-b][1,3,4]thiadiazole; microwave-assisted; synthesis; Cdc25B
Li Yingjun , Luo Tongchuan , Jin Kun , Gao Lixin , Shao Xin , Sheng Li , Yu Yang , Li Jia . Synthesis and Cdc25B Inhibition Activity of Novel Imidazo[2,1-b][1,3,4]thiadiazole Derivatives[J]. Chinese Journal of Organic Chemistry, 2014 , 34(2) : 325 -333 . DOI: 10.6023/cjoc201309013
[1] Lok, H. M.; Jessica, K.; Katherine, A. S.; Claire, S.; Gillian, F. W.; Yu, Y.; David, J. M.; Oscar, C.; James, S.; Rudiger, W. Bioorg. Med. Chem. 2012, 20, 4371.
[2] He, X. P.; Deng, Q.; Gao, L. X.; Li C.; Zhang, W.; Zhou, Y. B.; Tang, Y.; Shi, X. X.; Xie, J.; Li, J.; Chen, G. R.; Chen, K. X. Bioorg. Med. Chem. 2011, 19, 3892.
[3] Manal, S.; Diem, N. T.; Stéphanie, K.; Maria, A. M.; Bruno, O. V.; Christiane, G.; Emmanuelle, B. Bioorg. Med. Chem. Lett. 2012, 22, 7345.
[4] Andreani, A.; Burnelli, S.; Granaiola, M.; Leoni, A.; Locatelli, A.; Morigi, R.; Rambaldi, M.; Varoli, L.; Calonghi, N.; Cappadone, C.; Farruggia, G.; Zini, M.; Stefanelli, C.; Masotti, L.; Radin, N. S.; Shoemaker, R. H. J. Med. Chem. 2008, 51, 809.
[5] Karki, S. S.; Panjamurthy, K.; Raghavan, S. C.; Kumar, S.; Nambiar, M.; Ramareddy, S. A.; Chiruvella, K. K.; Raghavan, S. C. Eur. J. Med. Chem. 2011, 46, 2109.
[6] Noolvi, M. N.; Patel, H. M.; Singh, N.; Gadad, A. K.; Cameotra, S. S.; Badiger, A. Eur. J. Med. Chem. 2011, 46, 4411.
[7] Noolvi, M. N.; Patel, H. M.; Kamboj, S.; Kaur, A.; Kaur, A.; Mann, V. Eur. J. Med. Chem. 2012, 56, 56.
[8] Alegaon, S. G.; Alagawadi, K. R.; Sonkusare, P. V.; Chaudhary, S. M.; Dadwe, D. H.; Shah, A. S. Bioorg. Med. Chem. Lett. 2012, 22, 1917.
[9] Palkar, M. B.; Noolvi, M. N.; Maddi, V. S.; Ghatole, M.; Nargund, L. G. Med. Chem. Res. 2012, 21, 1313.
[10] Joshi, S. D.; Manish, K.; Aravind, B. Med. Chem. Res. 2013, 22, 869.
[11] Alagawadi, K. R.; Alegaon, S. G. Arabian J. Chem. 2011, 4, 465.
[12] Lamani, R. S.; Shetty, N. S.; Kamble, R. R.; Khazi, I. A. M. Eur. J. Med. Chem. 2009, 44, 2828.
[13] Bhardwaj, V.; Noolvi, M. N.; Jalhan, S.; Patel, H. M. J. Saudi Chem. Soc. 2013, doi: http://dx.doi.org/10.1016/j.jscs.2012.12.007.
[14] Dhepe, S.; Kumar, S.; Vinayakumar, R.; Ramareddy, S. A.; Karki, S. S. Med. Chem. Res. 2012, 21, 1550.
[15] Li, Y. J.; Liu, L. J.; Jin, K.; Sun, S. Q.; Xu, Y. T. Acta Chim. Sinica 2010, 68, 1577 (in Chinese).
(李英俊, 刘丽军, 靳焜, 孙淑琴, 许永廷, 化学学报, 2010, 68, 1577.)
[16] Li, Y. J.; Li, C. Y.; Jin, K.; Sun, S. Q.; Zhou, X. X. Acta Chim. Sinica 2012, 70, 151 (in Chinese).
(李英俊, 李春燕, 靳焜, 孙淑琴, 周晓霞, 化学学报, 2012, 70, 151.)
[17] Nie, C. M. J. Wuhan Univ. (Nat. Sci. Ed.) 2000, 46, 176 (in Chinese).
(聂长明, 武汉大学学报(自然科学版), 2000, 46, 176.)
[18] Li, Y. J.; Wang, J. K.; Xu, Y. T.; Li, L. N.; Li, C. Y.; Zhao, N. J. Liaoning Normal Univ. (Nat. Sci. Ed.) 2009, 23, 477 (in Chinese).
(李英俊, 王金奎, 许永廷, 李丽娜, 李春燕, 赵楠, 辽宁师范大学学报(自然科学版), 2009, 23, 477.)
[19] Organic Chemistry Research Groups, Department of Chemistry, Lanzhou University and Fudan University Experimental Organic Chemistry, People’s Education Press, Beijing, 1979, pp. 250~251 (in Chinese).
(兰州大学、复旦大学化学系有机化学教研组编, 有机化学实验, 人民教育出版社, 北京, 1979, pp. 250~251.)
[20] Geeta, L. R.; Bhushan, M. K. Synth. Commun. 2003, 33, 1405.
[21] Tang, Y. C.; Yu, S. X. Chem. Reag. 1995, 17, 384 (in Chinese).
(唐艳春, 于善信, 化学试剂, 1995, 17, 384.)
[22] Chen, P.; Xia, L. L.; Xiong, X. H.; Lu, F.; Yu, Q. CN 103012307, 2013 [Chem. Abstr. 2013, 158, 559852].
[23] Kulkarni M.; Kothawade, S.; Arabale, G.; Wagh, D.; Vijayamohanan, K.; Kulkarni, R. A.; Vernekar, S. P. Polymer 2005, 46, 3669.
[24] Li, Y. J.; Shao, X.; Gao, L. X.; Jin, K.; Sheng, L.; Luo, T. C.; Yu, Y.; Li, J. Chin. J. Org. Chem. 2013, 33, 2178 (in Chinese).
(李英俊, 邵昕, 高立信, 靳焜, 盛丽, 罗潼川, 于洋, 李佳, 有机化学, 2013, 33, 2178.)
/
| 〈 |
|
〉 |