

Chinese Journal of Organic Chemistry >
Synthesis and Biological Activities of 3-Methoxy-6-substituted-5,6-dihydropyrrolo[3,4-b]pyridin-7-ones
Received date: 2013-09-24
Revised date: 2013-10-14
Online published: 2013-11-20
Supported by
Project supported by the National Significant and Special Project of New Created Drugs (No. 2010ZX09401-404).
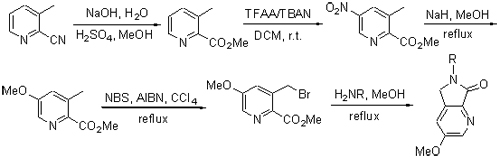
A series of thalidomide derivatives named 3-methoxy-6-substituted 5,6-dihydropyrrolo[3,4-b]pyridin-7-ones 1a~1l, which have never been reported in literature, were synthesized from 3-methyl pyridine-2-carboxylic acid methyl ester (7) and different amines by cyclization reaction. The intermediate 7 was produced via hydrolysis, esterification, nitration reaction, methoxy substitution, bromination reaction using 3-methyl-pyridine-2-carbonitrile as the starting material. The structures of all compounds have been confirmed by 1H NMR, 13C NMR and HRMS techniques. The target compounds were evaluated for their inhibitory activity against HCT-116, MG-63, MCF-7, HUVEC and HMVEC cells by MTT (thiazolyl blue tetrazolium bromide) method, and the results indicated that almost all of them had no obvious inhibitory effect on normal human cells, compounds 1h~1l only displayed obvious inhibitory effect on MG-63 cells while compounds 1c~1g presented excellent inhibitory activities against all the three kinds of tumor cells, among which compounds 1d and 1e exhibited most potent activities.
Sun Guanglong , Zhang Weiwei , Zhan Xiaoping , Liu Zenglu , Mao Zhenmin . Synthesis and Biological Activities of 3-Methoxy-6-substituted-5,6-dihydropyrrolo[3,4-b]pyridin-7-ones[J]. Chinese Journal of Organic Chemistry, 2014 , 34(3) : 546 -551 . DOI: 10.6023/cjoc201309033
[1] D'Amato, R. J.; Loughnan, M. S.; Flynn, E.; Folkman, J. Proc. Natl. Acad. Sci. U. S. A. 1994, 91, 4082.
[2] Dunzendorfer, S.; Schratzberger, P.; Reinisch, N.; Kahler, C. M.; Wiedermann, C. J. Naunyn. Schmiedeberg's Arch. Pharmacol. 1997, 356, 529.
[3] Richardson, P.; Hideshima, T.; Anderson, K. Annu. Rev. Med. 2002, 53, 629.
[4] Chen, L. H.; Li, D. D. Chin. J. New Drugs 2006, 15, 1141 (in Chinese).
(陈立慧, 李电东, 中国新药杂志, 2006, 15, 1141.)
[5] Greig, N. H.; Holloway, H.; Brossi, A.; Zhu, X. X.; Giordano, T.; Yu, Q. S.; Figg, W. D. WO 2005028436, 2005 [Chem. Abstr. 2005, 142, 355170].
[6] Miyachi, H.; Azuma, A.; Ogasawara, A.; Uchimura, E.; Watanabe, N.; Kobayashi, Y.; Kato, F.; Kato, M.; Hashimoto, Y. J. Med. Chem. 1997, 40, 2858.
[7] Man, H. W.; Corral, L. G.; Stirling, D. I.; Muller, G. W. Bioorg. Med. Chem. Lett. 2003, 13, 3415.
[8] Kotla, V.; Goel, S.; Nischal, S.; Heuck, C.; Vivek, K.; Das, B.; Verma, A. J. Hematol. Oncol. 2009, 2, 36.
[9] Kaplan, G.; Sampaio, E. P. US 5385901, 1992 [Chem. Abstr. 1992, 117, 226313].
[10] Kennenth, S. B.; Shannon, C. D.; William, D. F. Biochem. Pharmacol. 1998, 55, 1827.
[11] Fan, B.-L. Ph.D. Dissertation, Shanghai Jiaotong University, Shanghai, 2008 (in Chinese).
(范柏林, 博士论文, 上海交通大学, 上海, 2008.)
[12] Shi, Q.-F. M.S. Thesis, Shanghai Jiaotong University, Shanghai, 2012 (in Chinese).
(史群峰, 硕士论文, 上海交通大学, 上海, 2012.)
[13] Kale, R.; Tayade, P.; Saraf, M.; Juvekar, A. Drug Dev. Ind. Pharm. 2008, 34, 149.
[14] Shibata, Y.; Sasaki, K.; Hashimoto, Y.; Iwasaki, S. Chem. Pharm. Bull. 1996, 44, 156.
[15] Capitosti, S. M.; Hansen, T. P.; Brown, M. L. Bioorg. Med. Chem. 2004, 12, 327.
[16] Zhou, H. Ph.D. Dissertation, Shanghai Jiaotong University, Shanghai, 2013 (in Chinese).
(周恒, 博士论文, 上海交通大学, 上海, 2013.)
[17] Xie, Y.; Wu, G. Q.; Zheng, S. C.; Chen, F. E. Chin. J. Med. Chem. 2006, 16, 49 (in Chinese).
(谢艳, 吾国强, 郑士才, 陈芬儿, 中国药物化学杂志, 2006, 16, 49.)
[18] Zhang, W.-J.; Luo, Y.; Zhang, Y. M. Chin. J. Pharm. 2006, 37, 434 (in Chinese).
(张万金, 罗艳, 张燕梅, 中国医药工业杂志, 2006, 37, 434.)
[19] Njoroge, F. G.; Vibulbhan, B.; Pinto, P.; Chan, T. M.; Osterman, R.; Remiszewski, S.; Rosario, J. D.; Doll, R.; Girijavallabhan, V.; Ganguly, A. K. J. Org. Chem. 1998, 63, 445.
[20] Wang, Y.-H.; Song, Y. J.; Hu, Z.; Jing, J. H.; Meng, X. L.; Huang, Y. D. Chin. J. Org. Chem. 2009, 29, 780 (in Chinese).
(王艳红, 宋元军, 胡 桢, 景介辉, 孟祥丽, 黄玉东, 有机化学, 2009, 29, 780.)
[21] Xie, C.; Runnegar, M. T. C.; Snider, B. B. J. Am. Chem. Soc. 2000, 122, 5017.
[22] Clarke, K.; Goulding, J.; Scrowston, R. M. J. Chem. Soc., Perkin Trans. 1 1984, 1501.
[23] Zhang, E.; Li, C.; Zhang, B. Y.; Lü, A. Q.; Fang, Y.; Feng, S. Q.; Liu, H. M. Chin. J. Org. Chem. 2013, 33, 1100 (in Chinese).
(张恩, 李聪, 张保寅, 吕爱桥, 方园, 冯思琦, 刘宏民, 有机化学, 2013, 33, 1100.)
[24] Mamiko, S.; Yohei, M.; Hiroki, K.; Hiroyuki, K.; Aya, T.; Kazuo, N.; Yuichi, H. Chem. Pharm. Bull. 2003, 51, 1098.
[25] Caroline, S.; Jean-michel, R. J. Enzyme Inhib. Med. Chem. 2008, 23, 659.
[26] Yang, M.; He, J. B.; Cheng, Y. X.; Jiang, S. Chin. J. Org. Chem. 2013, 33, 1319 (in Chinese).
(杨梅, 何江波, 程永现, 蒋晟, 有机化学, 2013, 33, 1319.)
[27] Du, C.; Ren, Y. J.; Wang, Q. W.; Jin, L. Chin. J. Org. Chem. 2013, 33, 1279 (in Chinese).
(杜成, 任玉杰, 王庆伟, 金鹭, 有机化学, 2013, 33, 1279.)
/
| 〈 |
|
〉 |