

Chinese Journal of Organic Chemistry >
Synthesis and Bioactivity Studies of 2,3-Disubstituted Quinazolin-4(3H)-one
Received date: 2013-10-16
Revised date: 2013-11-13
Online published: 2013-11-21
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 20972186, 21172256), the National Basic Research Program of China (No. 2010CB126104) and the Project of Green Pesticide Research, Development and Industrialization (No. 2011BAE06B03).
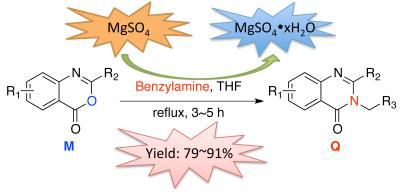
In this research, a series of new 2,3-disubstituted quinazolin-4(3H)-ones were designed and synthesized. The influence of common drying agents on the formation of 2,3-disubstituted quinazolin-4(3H)-ones was investigated and firstly reported. It is found that the anhydrous magnesium sulfate can increase the yield of 2,3-disubstituted quinazolin-4(3H)-ones significantly. The structures of new compounds were all confirmed via 1H NMR, 13C NMR and HRMS. At the same time, the crystal structure of 3-(benzylamino)-6-chloro-8-methyl-2-neopentyl-quinazolin-4(3H)-one was firstly reported. The results of bioactivity assay showed that some of these new compounds have some control effects on wheat powdery mildew (Blumeria graminis) in vivo at a concentration of 200 mg/L.
Ou Junjun , Liu Kechang , Wang Yi , Zhang Hao , Liu Ruiquan , Li Qibo , Wang Qingmin , Li Yongqiang , Rui Changhui , Liu Shangzhong . Synthesis and Bioactivity Studies of 2,3-Disubstituted Quinazolin-4(3H)-one[J]. Chinese Journal of Organic Chemistry, 2014 , 34(3) : 526 -536 . DOI: 10.6023/cjoc201310022
[1] Chen, H.-J.; Jiang, Y.-L.; Lin, C.-M. Int. J. Oncol. 2013, 43, 141.
[2] Patel, M. B.; Kumar, S. P.; Valand, N. N. J. Mol. Model. 2013, 19, 3201.
[3] Chao, Q.; Deng, L.; Shih, H. J. Med. Chem. 1999, 42, 3860.
[4] Beaulieu, P. L.; Coulombe, R.; Duan, J. Bioorg. Med. Chem. Lett. 2013, 23, 4132.
[5] Sahoo, B. M.; Dinda, S. C.; Kumar, B. V. V. R. Int. J. Pharm. Sci. Nanotechnol. 2013, 6, 2046.
[6] Abou-Seri, S. M.; Abouzid, K.; Abou El Ella, D. A. Eur. J. Med. Chem. 2011, 46, 647.
[7] Antonelli, A.; Fallahi, P.; Ferrari, S. M. Curr. Genomics 2011, 12, 626.
[8] Morgensztern, D.; Govindan, R. Chemother. Source Book, 4th ed., 2008, p. 191.
[9] (a) Zhou, J.; Fang, J. J. Org. Chem. 2011, 76, 7730.
(b) Sim, Y.-L.; Omer, N.; Khan, M. N. Tetrahedron 2013, 69, 2524.
(c) Chen, Y.; Shan, W.; Lei, M. Tetrahedron Lett. 2012, 53, 5923.
(d) Badri, R.; Alizadeh-Haddad, A.; Adlu, M. Bull. Chem. Soc. Ethiop. 2013, 27, 131.
[10] (a) Gao, X.; Cai, X.; Yan, K. Chin. J. Org. Chem. 2008, 28, 1785 (in Chinese).
(高兴文, 蔡学建, 严凯, 有机化学, 2008, 28, 1785.;
b) Gao, X.; Cai, X.; Yan, K. Molecules 2007, 12, 2621.
[11] Xie, M.; Chen, G.; Zou, L.Chem. Res. Appl. 2010, 864 (in Chinese).
(谢敏, 陈广民, 邹丽娟, 化学研究与应用, 2010, 864.)
[12] (a) Wang, X.; Li, P.; Li, Z. J. Agric. Food Chem. 2013, 61, 9575.
(b) Nanda, A. K.; Ganguli, S.; Chakraborty, R. Molecules 2007, 12, 2413.
(c) Ouyang, G.; Zhang, P.; Xu, G. Molecules 2006, 11, 383.
[13] (a) Liu, J.-F. Curr. Org. Synth. 2007, 4, 223.
(b) Besson, T.; Chosson, E. Comb. Chem. High Throughput Screening 2007, 10, 903.
(c) El-Mekabaty, A. Int. J. Mod. Org. Chem. 2013, 2, 81.
[14] Patil, D. A.; Patil, P. O.; Patil, G. B. Mini-Rev. Med. Chem. 2011, 11, 633.
[15] (a) Salehi, P.; Dabiri, M.; Zolfigol, M. A. Tetrahedron Lett. 2005, 41, 7051.
(b) Baghbanzadeh, M.; Dabiri, M.; Salehi, P. Heterocycles 2008, 11, 2809.
(c) Chen, J.; Wu, D.; He, F. Tetrahedron Lett. 2008, 23, 3814.
(d) Ishikawa, K.; Hosoe, T.; Itabashi, T. Sci. Pharm. 2011, 79, 937.
[16] O'Neil, M. J. The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals, 14th ed., Merck & Co., Inc., New Jersey, 2006, Monograph Number: 1658, 2653, 5691 and 8680.
[17] (a) Sati, N.; Kumar, S.; Rawat, M. S. M. Indian J. Pharm. Sci. 2009, 71, 572.
(b) Tseng, M.-C.; Chu, Y.-H. Tetrahedron 2008, 64, 9515.
[18] (a) Nerkar, A. G.; Kudale, S. A.; Joshi, P. P. Int. J. Pharm. Pharm. Sci. 2012, 4, 449.
(b) Chikhale, H.; Lade, K.; Joshi, P. Int. J. Pharm. Pharm. Sci. 2012, 4, 466.
[19] Armarego, W. L. F.; Chai, C. L. L. Purification of Laboratory Chemicals, 5th ed., Butterworth Heinemann Press, London, 2003, p. 361, p. 378.
[20] Muller, P.; Herbst, I. R.; Spek, A. L.; Schneider, T. R.; Sawaya, M. R. Crystal Structure Refinement—A Crystallographer's Guide to SHELXL, Oxford University Press, New York, 2006.
/
| 〈 |
|
〉 |