

Chinese Journal of Organic Chemistry >
Research Progress of Apratoxin A:A Marine Cyclicdepsipeptide with Significant Anti-cancer Activity
Received date: 2013-10-21
Revised date: 2013-12-04
Online published: 2013-12-06
Supported by
Project supported by the National Natural Science Foundation of China (No. 81001392, 81172975), the Project to Enhance the Research Ability of Young Teachers (Fudan University), and the National High Technology Research and Development Program of China (863 Program, No. 2013AA092903).
Apratoxin A, a marine natural cyclicdepsipeptide bearing novel and complex structure, showed significant antiproliferative activity against several cancer cell lines. A brief introduction on the research progress of this natural product including total synthesis, structure-activity relationship, pharmacological studies, and biosynthetic pathway was summarized.
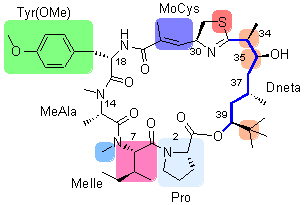
Key words: apratoxin A; marine bioactive peptide; anti-cancer
Zhang Wei , Liu Guoyun , Yin Ruwen , Li Yingxia . Research Progress of Apratoxin A:A Marine Cyclicdepsipeptide with Significant Anti-cancer Activity[J]. Chinese Journal of Organic Chemistry, 2014 , 34(3) : 475 -484 . DOI: 10.6023/cjoc201310033
[1] Newman, D. J.; Cragg, G. M.; Snader, K. M. J. Nat. Prod. 2003, 66, 1022.
[2] Wang, C.-Y.; Shao, C.-L. Marine Drugs, Science Press, Beijing, 2011, pp. 1~10 (in Chinese).
(王长云, 邵长伦, 海洋药物学, 科学出版社, 北京, 2011, pp. 1~10.)
[3] Luesch, H.; Yoshida, W. Y.; Moore, R. E.; Paul, V. J.; Corbett, T. H. J. Am. Chem. Soc. 2001, 123, 5418.
[4] Chen, J.; Forsyth, C. J. J. Am. Chem. Soc. 2003, 125, 8734.
[5] Chen, J.; Forsyth, C. J. Proc. Natl. Acad. Sci. U. S. A. 2004, 101, 12067.
[6] Doi, T.; Numajiri, Y.; Munakata, A.; Takahashi, T. Org. Lett. 2006, 8, 531.
[7] Ma, D.; Zou, B.; Cai, G.; Hu, X.; Liu, J. O. Chem. Eur. J. 2006, 12, 7615.
[8] Numajiri, Y.; Takahashi, T.; Doi, T. Chem. Asian J. 2009, 4, 111.
[9] Doi, T.; Numajiri, Y.; Takahashi, T.; Takagi, M.; Shin-ya, K. Chem. Asian J. 2011, 6, 180.
[10] Paterson, I.; Wallace, D. J.; Cowden, C. J. Synthesis 1998, 639.
[11] Chen, J.; Forsyth, C. J. Org. Lett. 2003, 5, 1281.
[12] Ma, D. 8th Chinese International Peptide Symposium, Kunming, 2004 (in Chinese).
(马大为, 第八届中国国际多肽学术会议, 昆明, 2004.)
[13] List, B.; Pojarliev, P.; Castello, C. Org. Lett. 2001, 3, 573.
[14] Raman, P.; Razavi, H.; Kelly, J. W. Org. Lett. 2000, 2, 3289.
[15] You, S.; Razavi, H.; Kelly, J. W. Angew. Chem., Int. Ed. 2003, 42, 83.
[16] Xu, Z.; Chen, Z.; Ye, T. Tetrahedron: Asymmetry 2004, 15, 355.
[17] Gilles, A.; Martinez, J.; Cavelier, F. C. R. Chim. 2011, 14, 437.
[18] Tidgewell, K.; Engene, N.; Byrum, T.; Media, J.; Doi, T.; Valeriote, F. A.; Gerwick, W. H. ChemBioChem 2010, 11, 1458.
[19] Thornburg, C. C.; Cowley, E. S.; Silorska, J.; Shaala, L. A.; Ishmael, J. E. Youssef, D. T. A.; McPhail, K. L. J. Nat. Prod. 2013, 76, 1781.
[20] Matthew, S.; Schupp, P. J.; Luesch, H. J. Nat. Prod. 2008, 71, 1113.
[21] Chen, Q.-Y.; Liu, Y.; Luesch, H. ACS Med. Chem. Lett. 2011, 2, 861.
[22] Luesch, H.; Yoshida, W. Y.; Moore, R. E.; Paul, V. J. Bioorg. Med. Chem. 2002, 10, 1973.
[23] Gutiérrez, M.; Suyama, T. L.; Engene, N.; Wignerd, J. S.; Matainaho, T.; Gerwick, W. H. J. Nat. Prod. 2008, 71, 1099.
[24] Luesch, H.; Chanda, S. K.; Raya, R. M.; Dejesus, P. D.; Orth, A. P.; Walker, J. R.; Belmonte, J. C. I.; Schultz, P. G. Nat. Chem. Biol. 2006, 2, 158.
[25] Shen, S.; Zhang, P.; Lovchik, M. A.; Li, Y.; Tang, L.; Chem, Z.; Zeng, R.; Ma, D.; Yuan, J.; Yu, Q. J. Cell Biol. 2009, 185, 629.
[26] Liu, Y.; Law, B. K.; Luesch, H. Mol. Pharm. 2009, 76, 91.
[27] Grindberg, R. V.; Ishoey, T.; Brinza, D.; Esquenazi, E.; Coates, R. C.; Liu, W.; Gerwick, L.; Dorrestein, P. C.; Pevzner, P.; Lasken, R.; Gerwick, W. H. PLoS One 2011, 6, e18565.
/
| 〈 |
|
〉 |