

Chinese Journal of Organic Chemistry >
Recent Advances in Chemical Synthesis of Backbone Cyclized Peptides
Received date: 2013-11-01
Revised date: 2013-11-19
Online published: 2013-12-06
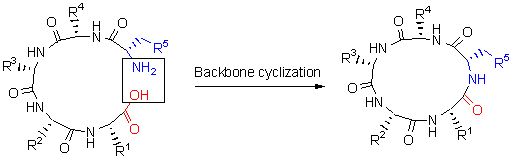
Backbone cyclized peptides belong to a family of circular polypeptide molecules in which the carboxyl and amino termini are covalently linked by an amide bond. Over the last almost 20 years, the backbone cyclized peptides have been discovered in bacteria, fungi, plants and animals. Compared with their linear precursors, the backbone cyclized peptides have a head-to-tail cyclic backbone enabling them to resist enzymatic degradation and to improve their thermal and chemical stability. Remarkably, some of them even have cell membrane permeability. Due to their exceptionally intracellular stability and potent bioactivities, backbone cyclized peptide is emerging as one of the most interesting molecules in the area of drug discovery. To study the structure and function of backbone cyclized peptides in detail, it is necessary to develop efficient approaches for the synthesis of these molecules. Herein, the recent advances in chemical synthesis of backbone cyclized peptides with respect to cyclization strategies are reviewed, including solid phase-based cyclization, liquid-phase cyclization, and intramolecular native chemical ligation.
Si Yanyan , Guo Ye , Li Haiyan , Sun Haoyuan , Fang Gemin . Recent Advances in Chemical Synthesis of Backbone Cyclized Peptides[J]. Chinese Journal of Organic Chemistry, 2014 , 34(3) : 450 -460 . DOI: 10.6023/cjoc201311001
[1] Scott, C. P.; Santos, E. A.; Wahnon, D. C.; Benkovic, S. J. Proc. Natl. Acad. Sci. U. S. A. 1999, 96, 13638.
[2] Kohli, R. M.; Walsh, C. T.; Burkart, M. D. Nature 2002, 418, 658.
[3] Tam, J. P.; Wong, C. T. T. J. Biol. Chem. 2012, 287, 27020.
[4] Morimoto, J.; Hayashi, Y.; Suga, H. Angew. Chem., Int. Ed. 2012, 51, 3423.
[5] Verdine, G. L.; Walensky, L. D. Clin. Cancer Res. 2007, 13, 7264.
[6] Pattabiraman, V. R.; Bode, J. W. Nature 2011, 480, 471.
[7] Cui, H.-K.; Guo, Y.; He, Y.; Wang, F.-L.; Chang, H.-N.; Wang, Y.-J.; Wu, F.-M.; Tian, C.-L.; Liu, L. Angew. Chem., Int. Ed. 2013, 52, 9558.
[8] Gause, G. F.; Brazhnikova, M. G. Nature 1944, 154, 703.
[9] Henriques, S. T.; Clark, D. T. Drug Discovery Today 2010, 15, 57.
[10] Camarero, J. A. Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 10025.
[11] Chin, J. W.; Schepartz, A. J. Am. Chem. Soc. 2001, 123, 2929.
[12] Davies, J. S. J. Pept. Sci. 2003, 9, 1502.
[13] Parenty, A.; Moreau, X.; Campagne, J. M. Chem. Rev. 2006, 106, 911.
[14] Jiang, S.; Li, Z.; Ding, K.; Roller, P. Curr. Org. Chem. 2008, 12, 1502.
[15] Clark, R. J.; Craik, D. J. Biopolymers 2010, 94, 414.
[16] White, C. J.; Yudin, A. K. Nat. Chem. 2011, 3, 509.
[17] Wang, D. X.; Hang, X.; Gong, X.; Feng, H. H. Chin. J. Org. Chem. 2008, 28, 549.
[18] Alcaro, M. C.; Sabatino, G.; Uziel, J.; Chelli, M.; Ginanneschi, M.; Rovero, P.; Papini, A. M. J. Pept. Sci. 2004, 10, 218.
[19] Sabatino, G.; Chelli, M.; Mazzucco, S.; Ginanneschi, M.; Papini, A. M. Tetrahedron Lett. 1999, 40, 809.
[20] Alsina, J.; Rabanal, F.; Giralt, E.; Albericio, F. Tetrahedron Lett. 1994, 35, 9633.
[21] Bolscher, J. G. M.; Oudhoff, M. J.; Nazmi, K.; Antos, J. M.; Guimaraes, C. P.; Spooner, E.; Haney, E. F.; Vallejo, J. J. G.; Vogel, H. J.; Hof, W. V.; Ploegh, H. L.; Veerman, E. C. I. FASEB J. 2011, 25, 2650.
[22] Yang, L.; Morriello, G. Tetrahedron Lett. 1999, 40, 8197.
[23] Bourel-Bonnet, L.; Rao, K. V.; Hamann, M, T.; Ganesan, A. J. Med. Chem. 2005, 48, 1330.
[24] de Visser, P. C.; Kriek, N. M.; van Hooft, P. A.; Filippov, D. V.; van der Marel, G. A.; Overkleeft, H. S.; van Boom, J. H.; Noort, D. J. J. Pept. Res. 2003, 61, 298.
[25] Mukhtar, T. A.; Koteva, K. P.; Wright, G. D. Chem. Biol. 2005, 12, 229.
[26] Ali, L.; Musharraf, S. G.; Shaheen, F. J. Nat. Prod. 2008, 71, 1059.
[27] Wieland, T.; Lewalter, J.; Birr, C. Liebigs Ann. Chem. 1970, 31, 740.
[28] Schmidt, U.; Langner, J. J. Pept. Res. 1997, 49, 67.
[29] Rosenbaum, C.; Waldmann, H. Tetrahedron Lett. 2001, 42, 5677.
[30] Shigenaga, A.; Moss, J. A.; Ashley, F. T.; Kaufmann, G. F.; Janda, K. D. Synlett 2006, 551.
[31] Camarero, J. A.; Hackel, B. J.; de Yoreo, J. J.; Mitchell, A. R. J. Org. Chem. 2004, 69, 4145.
[32] Aboye, T. L.; Li, Y.-L.; Majumder, S.; Hao, J.-F.; Alexander Shekhtman, J. A. Camarero, J. A. Bioorg. Med. Chem. Lett. 2012, 22, 2823.
[33] Heinlein, C.; Silva, D. V.; Troster, A.; Schmidt, J.; Gross, A.; Unverzagt, C. Angew. Chem., Int. Ed. 2011, 50, 6406.
[34] Rückle, T.; de Lavallaz, P.; Keller, M.; Dumy, P.; Mutter, M. Tetrahedron 1999, 55, 11281.
[35] Skropeta, D.; Jolliffe, K. A.; Turner, P. J. Org. Chem. 2004, 69, 8804.
[36] Fairweather, K. A.; Sayyadi, N.; Luck, I. J.; Clegg, J. K.; Jolliffe, K. A. Org. Lett. 2010, 12, 3136.
[37] Ye, Y.-H.; Gao, X.-M.; Liu, M.; Tang, Y.-C; Tian, G.-L. Lett. Pept. Sci. 2003, 10, 571.
[38] Liu, M.; Tang, Y. C.; Fan, K. Q.; Jiang, X.; Lai, L. H.; Ye, Y. H. J. Pept. Res. 2005, 65, 55.
[39] Sasaki, K.; Crich, D. Org Lett. 2010, 12, 3254.
[40] Crich, D.; Sharma, I. Angew. Chem., Int. Ed. 2009, 48, 7591.
[41] Crich, D.; Sharma, I. Angew. Chem., Int. Ed. 2009, 48, 2355.
[42] Aimoto, S.; Mizoguchi, N.; Hojo, H.; Yoshimura, S. Bull. Chem. Soc. Jpn. 1989, 62, 524.
[43] Zhang, L.-S.; Tam, J. P. Tetrahedron Lett. 1997, 38, 4575.
[44] Li, Y.; Yongye, A.; Giulianotti, M.; Martinez-Mayorga, K.; Yu, Y.; Houghten, R. A. J. Comb. Chem. 2009, 11, 1066.
[45] Li, Y.; Giulionatti, M.; Houghten, R. A. Org. Lett. 2010, 12, 2250.
[46] Nilsson, B. L.; Kiessling, L. L.; Raines, T. R. Org. Lett. 2000, 2, 1939.
[47] Saxon, E. J. I.; Armstrong, J. I.; Bertozzi, C. R. Org. Lett. 2000, 2, 2141.
[48] Kleineweischede, R.; Hackenberger, C. P. R. Angew. Chem., Int. Ed. 2008, 47, 5984.
[49] Liu, C.-F.; Tam, J. P. Proc. Natl. Acad. Sci. U. S. A. 1994, 91, 6584.
[50] Li, X.-C.; Lam, H.-Y.; Zhang, Y.-F.; Chan, C.-K. Org. Lett. 2010, 12, 1724.
[51] Lam, H.-Y.; Zhang, Y.-F.; Liu, H.; Xu, J.-C.; Wong, C. T. T.; Xu, C.; Li, X.-C. J. Am. Chem. Soc. 2013, 135, 6272.
[52] Wong, C. T. T.; Lam, H.-Y.; Song, T.; Chen, G.-H.; Li, X.-C. Angew. Chem., Int. Ed. 2013, 52, 10212.
[53] Bode, J. W.; Fox, R. M.; Baucom, K. D. Angew. Chem., Int. Ed. 2006, 45, 1248.
[54] Fukuzumi, T.; Ju, L.; Bode, J. W. Org. Biomol. Chem. 2012, 10, 5837.
[55] Dawson, P. E.; Muir, T. W.; Clark-Lewis, I.; Kent. S. B. Science 1994, 266, 776.
[56] Kent, S. B. H. Chem. Rev. Soc. 2009, 38, 338.
[57] Torbeev, V. Y.; Kent, S. B. H. Angew. Chem., Int. Ed. 2007, 46, 1667.
[58] Sohma, Y.; Kent, S. B. H. J. Am. Chem. Soc. 2009, 131, 16313.
[59] Bang, D.; Pentelute, B. L.; Kent, S. B. H. Angew. Chem., Int. Ed. 2006, 45, 3985.
[60] Mandal, K.; Kent, S. B. H. Angew. Chem., Int. Ed. 2011, 50, 8029.
[61] Huang, Y.-C.; Li, Y.-M.; Chen, Y.; Pan, M.; Li, Y.-T.; Yu, L.; Guo, Q.-X.; Liu, L. Angew. Chem., Int. Ed. 2013, 52, 4858.
[62] Tam, J. P.; Lu, Y. A. Tetrahedron Lett. 1997, 38, 5599.
[63] Tam, J. P.; Lu, Y. A.; Yu, Q. J. Am. Chem. Soc. 1999, 121, 4316.
[64] Tam, J. P.; Wong, C. T. J. Biol. Chem. 2012, 287, 27020.
[65] Warren, J. D.; Miller, J. S.; Keding, S. J.; Danishefsky, S. J. J. Am. Chem. Soc. 2004, 126, 6576.
[66] Chen, J.; Warren, J. D.; Wu, B.; Chen, G.; Wan, Q.; Danishefsky, S. J. Tetrahedron Lett. 2006, 47, 1969.
[67] Botti, P.; Villain, M.; Manganiello, S.; Gaertner, H. Org. Lett. 2004, 6, 4861.
[68] Zheng, J.-S.; Cui, H.-K.; Fang, G.-M.; Xi, W.-X.; Liu. L. ChemBioChem 2010, 11, 511.
[69] Fang, G.-M.; Cui, H.-K.; Zheng, J.-S.; Liu. L. ChemBioChem 2010, 11, 1061.
[70] Zheng, J.-S.; Chang, H.-N.; Shi, J.; Liu, L. Sci. China Chem. 2012, 55, 64.
[71] Macmillan, D.; Cecco, M. D.; Reynolds, N. L. Santos, L. F. A.; Barran, P. E.; Dorin, J. R. ChemBioChem 2011, 12, 2133.
[72] Taichi, M.; Hemu, X.; Qiu, Y.-B.; Tam, J. P. Org. Lett. 2013, 15, 2620.
[73] Fang, G.-M.; Li, Y.-M.; Shen, F.; Huang, Y.-C.; Li, J.-B.; Lin, Y.; Cui, H.-K.; Liu, L. Angew. Chem., Int. Ed. 2011, 50, 7645.
[74] Fang, G.-M.; Wang, J.-X.; Liu, L. Angew. Chem., Int. Ed. 2012, 51, 10347.
[75] Zheng, J.-S.; Chang, H.-N.; Wang, F.-L.; Liu, L. J. Am. Chem. Soc. 2011, 133, 11080.
[76] Liang, J.; Fang, G.-M.; Huang, X.-L.; Mei, Z.-Q.; Li, J.; Tian, C.-L.; Liu, L. Sci. China Chem. 2013, 56, 1301.
[77] Zheng, J.-S.; Huang, Y.-C.; Tang, S.; Liu, L. Acc. Chem. Res. 2013, 2013, 46, 2475.
[78] Zheng, J.-S.; Tang, S.; Qi, Y.-K.; Wang, Z.-P.; Liu, L. Nat. Protoc. 2013, 8, 2483.
[79] Li, Y.-M.; Yang, M.-Y.; Huang, Y.-C.; Li, Y.-T.; Chen, P.-R.; Liu, L. ACS Chem. Biol. 2012, 7, 1015.
[80] Zheng, J.-S.; Tang, S.; Guo, Y.; Chang, H.-N.; Liu, L. ChemBioChem 2012, 13, 542.
[81] Tang, S.; Zheng, J.-S.; Yang, K.; Liu, L. Acta Chim. Sinica 2012, 70, 1471.
[82] Wong, C. T. T.; Rowlands, D. K.; Wong, C.-F.; Lo, T. W. C.; Nguyen, G. K. T.; Li, H.-Y.; Tam, J. P. Angew. Chem., Int. Ed. 2012, 51, 5620.
[83] Ji, Y.-B.; Majumder, S.; Millard, M.; Borra, R.; Bi, T.; Elnagar, A. Y.; Neamati, N.; Shekhtman, A.; Camarero, J. A. J. Am. Chem. Soc. 2013, 135, 11623.
/
| 〈 |
|
〉 |