

Chinese Journal of Organic Chemistry >
Synthesis and Fungicidal Activity of Methyl 2-Methoxyimino-2-polysubstituted-phenylacetates
Received date: 2013-09-02
Revised date: 2013-12-02
Online published: 2013-12-13
Supported by
Project supported by the National “Twelfth Five-Year” Plan for Science & Technology Support (Nos. 2011BAE06B03-12, 2011BAE06B05), the National Natural Science Foundation of China (No. 21172114) and the Natural Science Foundation of Tianjin City (No. 11JCYBJC14200).
Reaction of methyl 2-chloro-2-oxacetate and 4-chlorotoluene (or 3-tert-butyltoluene) in the presence of AlCl3 in CH2Cl2 generated 2-(5-chloro-or 4-tert-butyl-toluene)-2-methyl-phenyl-2-oxoacetate (A), which were treated with O-methyl-hydroxylamine to furnish (Z/E)-methyl-2-(5-chloro (or 4-tert-butyl)-2-methyl phenyl)-2-(methoxyimino)acetates (B). B were brominated with Br2 to afford the intermediate (Z/E)-ethyl-2-(2-bromomethyl-5-chloro (or 4-tert-butyl)-2-methyl-phenyl)-2-(methoxyimino)acetates (C). The intermediate E-methyl-2-(2-(bromomethyl phenyl)-5-nitro-phenyl)-2-(methoxyimino)acetate (F) was obtained from nitration of E-methyl-2-(2-(bromomethyl)phenyl)-2-(methoxyimino)acetate (E). The intermediates C and F were condensed with various aryl ketoximes to produce the title compounds (D, E and G). Compound H was obtained from the reduction of G1. All of the title compounds have been characterized by 1H NMR, 13C NMR, 19F NMR, IR and HRMS. All of the title compounds were tested for fungicidal activities against cucumber gray mold, tomato disease early, wheat gibberellic, pepper phytophthora, rape sclerotium, rice grain dry and so forth by the mycelium growth rate method, which indicated that some of them displayed better fungicidal activity than that of trifloxystrobin.
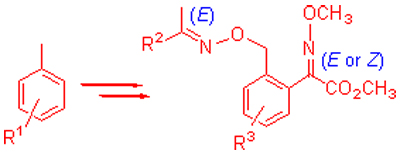
Key words: methoxyl acrylate compounds; synthesis; fungicidal activity
Jiang Wentao , Hu Fangzhong , Gu Han , Liu Chuan , Wei Naixiang , Wan Lei , Ren Shizhao , Wang Junting , Xu Fengbo . Synthesis and Fungicidal Activity of Methyl 2-Methoxyimino-2-polysubstituted-phenylacetates[J]. Chinese Journal of Organic Chemistry, 2014 , 34(4) : 774 -782 . DOI: 10.6023/cjoc201309004
/
| 〈 |
|
〉 |