

Chinese Journal of Organic Chemistry >
Application of Bipyridyl Complexes in Coupling Reactions
Received date: 2013-09-27
Revised date: 2013-11-19
Online published: 2013-12-13
Supported by
Project supported by the Natural Science Foundation of Shanxi Province (Nos. 2012021007-2, 2011011010-2) and the Scientific and Technological Innovation Programs of Higher Education Institutions in Shanxi Province (No. 20120006).
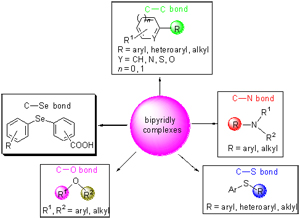
The bipyridyl ligands have been extensively used as metal chelating complexes or directly utilized as ligands due to their robust redox stability and ease of functionalization which also become more and more important in the application of the organic synthesis. This article mainly describes the application of bipyridyl complexes in coupling reactions for the formation of C—C, C—N, C—S, C—O and C—Se bonds.
Li Yingjun , Li Zhipeng , Li Xing , Chang Honghong , Wei Wenlong . Application of Bipyridyl Complexes in Coupling Reactions[J]. Chinese Journal of Organic Chemistry, 2014 , 34(4) : 693 -705 . DOI: 10.6023/cjoc201309039
[1] Kawano, T.; Shinomaru, T.; Ueda, I. Org. Lett. 2002, 4, 2545.
[2] Sinner, F.; Buchmeiser, M. R.; Tessadri, R.; Mupa, M.; Wurst, K.; Bonn, G. K. J. Am. Chem. Soc. 1998, 120, 2790.
[3] Kaes, K.; Katz, K.; Hosseini, W. Chem. Rev. 2000, 100, 3553.
[4] Beccalli, E. M.; Broggini, G.; Martinelli, M.; Sottocornola, S. Chem. Rev. 2007, 107, 5318.
[5] Turner, N. J. Chem. Rev. 2011, 111, 4073.
[6] Beletskaya, I. P.; Ananikov, V. P. Chem. Rev. 2011, 111, 1596.
[7] Ullmann, F. Ber. Dtsch. Chem. Ges. 1903, 36, 2382.
[8] Zhang, B. B.; Zhan, D.; Zhang, X. P.; Xiang, Q. J.; Zeng, Q. L. Acta Chim. Sinica 2012, 70, 1655 (in Chinese). (张斌彬, 詹丹, 张小平, 向沁洁, 曾庆乐, 化学学报, 2012, 70, 1655.)
[9] Li, X.; Yan, X. Y.; Chang, H. H.; Wang, L. C.; Zhang, Y.; Chen, W. W.; Li, Y. W.; Wei, W. L. Org. Biomol. Chem. 2012, 10, 495.
[10] Li, X.; Wang, L. C.; Chang, H. H.; Zhang, C. X.; Wei, W. L. Appl. Catal. A: Gen. 2013, 462~463, 15.
[11] Yin, J.; Rainka, M. P.; Zhang, X. X.; Buchwald, S. L. J. Am. Chem. Soc. 2002, 124, 1162.
[12] Matthias, A.; Oberli, M. A.; Stephen, L.; Buchwald, S. L. Org. Lett. 2012, 14, 4606.
[13] Jiang, L.; Li, Z. N.; Zhao, D. F. Acta Chim. Sinica 2010, 30, 200 (in Chinese). (姜岚, 李争宁, 赵德峰, 化学学报, 2012, 30, 200.)
[14] Paddock, R. L.; Nguyen, S. T. J. Am. Chem. Soc. 2001, 123, 11498.
[15] Mizoroki, T.; Mori, K.; Ozaki, A. Bull. Chem. Soc. Jpn. 1971, 44, 581.
[16] Heck, R. F.; Nolley, J. P. J. Org. Chem. 1972, 14, 2320
[17] Nájera, C.; Gil-Moltó, J.; Karlstrёm, S.; Falvello, L. R. Org. Lett. 2003, 5, 1451.
[18] Tsai, F. Y.; Wu, C. L.; Mou, C. Y.; Chao, M. C.; Lin, H. P.; Liu, S. T. Tetrahedron Lett. 2004, 45, 7503.
[19] Gil-Moltó, J.; Karlstrёm, S.; Nájera, C. Tetrahedron 2005, 61, 12168.
[20] Huang, S. H.; Chen, J. R.; Tsai, F. Y. Molecules 2010, 15, 315.
[21] Jatap, S. V.; Deshpande, R. M. Kinet. Catal. 2013, 54, 314.
[22] Miyauar, N.; Suzuki, A. Chem. Rev. 1995, 95, 2457.
[23] Suzuki, A. Organomet. Chem. 1999, 576, 147.
[24] Nájera, C.; Gil-Moltó, J.; Karlstrom, S. Adv. Synth. Catal. 2004, 346, 1798.
[25] Wu, W. Y.; Chen, S. N.; Tsai, F. Y. Tetrahedron Lett. 2006, 47, 9267.
[26] Osako, T.; Uozumi, Y. Heterocycles 2010, 80, 505.
[27] Huang, J. P.; Wang, W.; Li, H. X. ACS Catal. 2013, 3, 1526.
[28] Negishi, E. I.; Baba, S. J. Chem. Soc., Chem. Commun. 1976, 15, 596.
[29] Baba, S.; Negishi, E. I. J. Am. Chem. Soc. 1976, 98, 6729.
[30] Wu, W. Y.; Lin, T. C.; Takahashi, T.; Tsai, F. Y.; Mou, C. Y. ChemCatChem 2013, 5, 1011.
[31] Hatanaka, Y.; Hiyama, T. J. Org. Chem. 1988, 53, 918.
[32] Chen, S. N.; Wu, W. Y.; Tsai, F. Y. Tetrahedron 2008, 64, 8164.
[33] Corriu, R. J. P.; Masse, J. P. J. Chem. Soc., Chem. Commun. 1972, 3, 144.
[34] Tamao, K.; Sumitani, K.; Kumada, M. J. Am. Chem. Soc. 1972, 94, 4374.
[35] Tsai, F. Y.; Lin, B. N.; Chen, M. J.; Mou, C. Y.; Liu, S. T. Tetrahedron 2007, 63, 4304.
[36] Sonogashira, K. Organomet. Chem. 2002, 653, 46.
[37] Gil-Moltó, J.; Nájera, C. Eur. J. Org. Chem. 2005, 19, 4073.
[38] Lin, B. N.; Huang, S. H.; Wu, W. Y.; Mou, C. Y.; Tsai, F. Y. Molecules 2010, 15, 9157.
[39] Hung, T. T.; Huang, C. M.; Tsai, F. Y. ChemCatChem 2012, 4, 540.
[40] Fang, J. H.; Hu, M. J.; Wang, J. R.; Fu, Z. Q. J. Mol. Catal. 2006, 20, 335 (in Chinese). (房江华, 胡敏杰, 王家荣, 付志强, 分子催化, 2006, 20, 335.)
[41] Wang, Y. H.; Tsai, F. Y. Chem. Lett. 2007, 36, 1492.
[42] Chen, S. N.; Wu, W. Y.; Tsai, F. Y. Green Chem. 2009, 11, 269.
[43] Wu, T. M.; Huang, S. H.; Tsai, F. Y. Appl. Organomet. Chem. 2011, 25, 395.
[44] Yamamoto, T. Chem. Lett. 2012, 41, 1422.
[45] Chen, J. Y.; Chen, S. C.; Tang, Y. J.; Mou, C. Y.; Tsai, F. Y. J. Mol. Catal. A: Chem. 2009, 307, 88.
[46] Chen, J. Y.; Lin, T. C.; Chen, S. C.; Chen, A. J.; Mou, C. Y.; Tsai, F. Y. Tetrahedron 2009, 65, 10134.
[47] Nishihara, Y.; Ogawa, D.; Noyori, S.; Iwasaki, M. Chem. Lett. 2012, 41, 1503.
[48] Kohls, P.; Jadhav, D.; Pandey, G.; Reiser, O. Org. Lett. 2012, 14, 672.
[49] Savmarker, J.; Rydfjord, J.; Gising, J.; Odell, L. R.; Larhed, M. Org. Lett. 2012, 14, 2393.
[50] Yasu, Y.; Koike, T.; Akita, M. Chem. Commun. 2012, 48, 5355.
[51] Gao, X. W.; Meng, Q. Y.; Xing, M.; Chen. B.; Feng, K.; Tung, C. H.; Wu, L. Z. Adv. Synth. Catal. 2013, 355, 2158.
[52] Yi, C. Q.; Cheng, J.; Zhang, P. Chen, K. J. Wuhan Inst. Technol. 2013, 35, 1 (in Chinese). (尹传奇, 成军, 张平, 陈阔, 武汉工程大学学报, 2013, 35, 1.)
[53] Liang, L.; Li, Z. K.; Zhou, X. G. Org. Lett. 2009, 11, 3294.
[54] Srimani, D.; Balaraman, E.; Hu, P.; Yehoshoa, B. D.; Milstein, D. Adv. Synth. Catal. 2013, 355, 2525.
[55] Lanke, S. R.; Bhanage, B. M. Appl. Organomet. Chem. 2013, 27, 729.
[56] Wu, W. Y.; Wang, J. C.; Tsai, F. Y. Green Chem. 2009, 11, 326.
[57] Lan, M. T.; Wu, W. Y.; Huang, S. H.; Luo, K. L.; Tsai, F. Y. RSC Adv. 2011, 1, 1751.
[58] Wang, X.; Cuny, G. D.; Noёl, T. Angew. Chem., Int. Ed. 2013, 52, 7860.
[59] Koike, T.; Akita, M. Chem. Lett. 2009, 38, 166.
[60] Phan, N. T. S.; Vu, P. H. L.; Nguyen, T. T. J. Catal. 2013, 306, 38.
[61] Phan, N. T. S.; Nguyen, T. T.; Vu, P. H. L. ChemCatChem 2013, 5, 3068. Millois, C.; Diaz, P. Org. Lett. 2000, 2, 1705.
/
| 〈 |
|
〉 |