

Chinese Journal of Organic Chemistry >
Synthesis of Novel Trichloride Indenyl Zirconium Complexes with Pendant Pyridine Moiety
Received date: 2013-10-21
Revised date: 2013-12-05
Online published: 2013-12-19
Supported by
Project supported by the National Natural Science Foundation of China (No. 20372022).
It is always difficult to synthesize half-sandwich trichloride cyclopentadienyl (indenyl) zirconium complexes due to accompanying the formation of sandwich metallocene (indenyl) zirconium complexes. In this paper, a series of novel trichloride indenyl zirconium complexes, [Ind-Bridge-Py]ZrCl3·THFx (CN1~CN3), bearing pendant pyridine moiety with chelating ring structure, were synthesized selectively by directly reacting lithium salts of substituted indenyl with ZrCl4·2THF, and no di-indenyl zirconium complexes were formed. Compared with conventional methods of synthesizing half-sandwich indenyl complexes, the synthetic method of these kinds of trichloride indenyl zirconium complexes with pendant pyridine moiety has the advantages of easy operation, single product, easy separation and high yield. The X-ray of CN2 [Bridge: C(Me, Et)CH2] shows that zirconium is coordinated with N atom and also with one O atom from THF. Another molecule of THF in the crystal is free and not coordinated with zirconium.
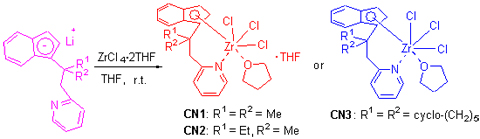
Key words: pyridine; trichloride indenyl zirconium; coordination; tetrahydrofuran
Zhang Yanlu , Ma Haiyan , Huang Jiling . Synthesis of Novel Trichloride Indenyl Zirconium Complexes with Pendant Pyridine Moiety[J]. Chinese Journal of Organic Chemistry, 2014 , 34(1) : 197 -203 . DOI: 10.6023/cjoc201310032
[1] Schellenberg, J. Prog. Polym. Sci. 2009, 34, 688.
[2] Zhang, Y. L.; Ma, H. Y.; Huang, J. L. J. Mol. Catal. A: Chem. 2013, 373, 85.
[3] McGuinness, D. S. Chem. Rev. 2011, 111, 2321.
[4] Deckers, P. J. W.; Hessen, B.; Teuben, J. H. Angew. Chem., Int. Ed. 2001, 40, 2516.
[5] Huang, J. L.; Wu, T. Z.; Qian, Y. L. Chem. Commun. 2003, 2816.
[6] Suttil, J. A.; McGuinness, D. S.; Evans, S. J. Dalton. Trans. 2010, 39, 5278.
[7] Lund, E. C.; Livinghouse, T. Organometallics 1990, 9, 2426.
[8] Sieb, D.; Schuhen, K.; Morgen, M.; Herrmann, H.; Wadepohl, H.; Lucas, N. T.; Baker, R. W.; Enders, M. Organometallics 2012, 31, 356.
[9] Dreier, T.; Fröhlich, R.; Erker, G. J. Organomet. Chem. 2001, 621, 197.
[10] Shaw, S. L.; Morris, R. J.; Huffman, J. C. J. Organomet. Chem. 1995, 489, C4.
[11] Allan, L. E. N.; Clarkson, G. J.; Fox, D. J.; Gott, A. L.; Scott, P. J. Am. Chem. Soc. 2010, 132, 15308.
[12] Saβmannshausen, J.; Powell, A. K.; Anson, C. E.; Wocadlo, S.; Bochmann, M. J. Organomet. Chem. 1999, 592, 84.
[13] Manna, K.; Everett, W. C.; Schoendorff, G.; Ellern, A.; Windus, T. L.; Sadow, A. D. J. Am. Chem. Soc. 2013, 135, 7235.
[14] Tagge, C. D.; Kravchenko, R. L.; Lal, T. K.; Waymouth, R. M. Organometallics 1999, 18, 380.
[15] Krut'ko, D. P. Russ. Chem. Bull., Int. Ed. 2009, 58, 1745.
[16] Krut'ko, D. P.; Belov, S. A., Kirsanov, R. S.; Lemenovskii, D. A., Churakov, A. V. Russ. Chem. Bull., Int. Ed. 2010, 59, 329.
[17] Ma, H. Y.; Huang, J. L.; Qian, Y. L. Inorg. Chem. Commun. 2001, 4, 515.
[18] Zhang, H.; Ma, J. Qian, Y. L.; Huang, J. L. Organometallics 2004, 23, 5681.
[19] Liu, K. F.; Wu, Q. L.; Luo, X. Y.; Gao, W.; Mu, Y. Dalton Trans. 2012, 41, 3461.
[20] Lei, Y. L.; Wang, Y. B.; Luo, Y. J. J. Organometal. Chem. 2013, 738, 24.
[21] Wang, Y. B.; Lei, Y. L.; Chi, S. H.; Luo, Y. J. Dalton Trans. 2013, 42, 1862.
[22] Guan, S. Z.; Nie, W. L.; Borzov, M. V. Acta Crystallogr. 2011, 67, 540.
[23] Krut'ko, D. P.; Kirsanov, R. S.; Belov, S. A.; Borzov, M. V.; Churakov, A. V. J. Organomet. Chem. 2007, 692, 1465.
[24] Ziniuk, Z.; Goldberg, I.; Kol, M. J. Organomet. Chem. 1997, 545~546, 441.
[25] Enders, M.; Rudolph, R.; Pritzkow, H. Chem. Ber. 1996, 129, 459.
/
| 〈 |
|
〉 |