

Chinese Journal of Organic Chemistry >
A Novel and Green Method for the Synthesis of 4-Thiazolidinones with the Sources of 2-Chloroacetamide and Thioureas
Received date: 2013-10-25
Revised date: 2013-12-05
Online published: 2013-12-19
Supported by
Project supported by the Natural Science Foundation of Zhejiang Province (Nos. LY12B02015, Y4080234).
A novel, green synthetic method for 4-thiazolidinones in the ionic liquids was reported. 4-Thiazolidinones were prepared with the sources of substituted thioureas and 2-chloroacetamide in the ionic liquids. This method offers several advantages like high yield, short reaction time and mild reaction conditions. The reaction mechanism was studied and the result showed that ammonia was removed in the reaction, which was the first reported.
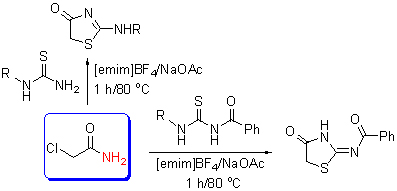
Key words: 4-thiazolidinones; 2-chloroacetamide; ionic liquid; mechanism; green
Zhao Huarong , Wang Ling , Wang Yudan . A Novel and Green Method for the Synthesis of 4-Thiazolidinones with the Sources of 2-Chloroacetamide and Thioureas[J]. Chinese Journal of Organic Chemistry, 2014 , 34(4) : 761 -766 . DOI: 10.6023/cjoc201310038
/
| 〈 |
|
〉 |