

Chinese Journal of Organic Chemistry >
Synthesis and AChE Inhibitory Activity of Chalcones Mannich Base Derivatives
Received date: 2013-11-14
Revised date: 2013-12-18
Online published: 2013-12-23
Supported by
Project supported by the Hunan Provincial Natural Science Foundation of China (No. 14JJ2048) and the National Natural Science Foundation of China (No. 21342015).
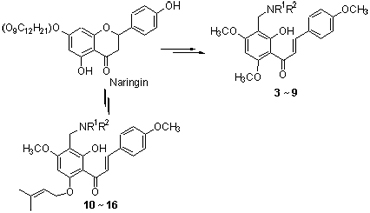
Chalcone flavokawain A (1) and chalcone prenyl ether derivative 2 were synthesized from naringin, through glycoside hydrolysis, O-methylation or O-prenylation, and base-catalyzed ring-opening reaction. Based on Mannich reaction of chalcone 1 and chalcone prenyl ether derivative 2, fourteen new chalcone Mannich base derivatives 3~16 were synthesized. The aminomethylation occurred preferentially at 3'-C position of chalcones. The structures of all synthesized compounds were determined by MS, NMR and IR spectra. All the synthetic compounds were evaluated for acetylcholinesterase (AChE) inhibitory activity. The results show that chalcone mannich base derivatives 3~5, 9 exhibit good AChE inhibitory activity.
Xu Shouhui , Liu Haoran , Lou Dinghui , Wang Qiuan . Synthesis and AChE Inhibitory Activity of Chalcones Mannich Base Derivatives[J]. Chinese Journal of Organic Chemistry, 2014 , 34(4) : 749 -755 . DOI: 10.6023/cjoc201311025
/
| 〈 |
|
〉 |