

Chinese Journal of Organic Chemistry >
Recent Advances on Multicomponent Reactions Based on the Transamination Process of Electron Deficient Enamines
Received date: 2013-12-15
Revised date: 2014-01-05
Online published: 2014-01-10
Supported by
Project supported by the National Natural Science Foundation of China (No. 21102059), the Project from the Department of Education of Jiangxi Province (No. GJJ13245) and the Sponsored Program for Cultivating Youths of Outstanding Ability in Jiangxi Normal University.
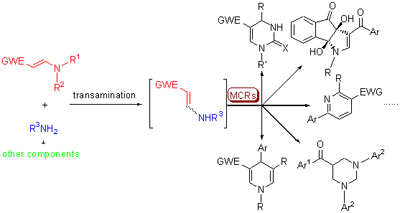
Electron deficient enamines bear several different nucleophilic and eletrophilic sites in their structures, they can also serve as donors of C=C bonds in organic reactions. Therefore, electron deficient enamines have been broadly employed in organic synthesis via various nucleophilic, electrophilic transformation and cycloaddition. Based on the research interest and works of our group in the chemistry of electron deficient enamines, the research advances on multicomponent reactions (MCRs) are reviewed based on the transamination of electron deficient enamines with ammomium, amines, enamines, ureas and thioureas as key transformation.
Cao Shuo , Jing Yanfeng , Liu Yunyun , Wan Jieping . Recent Advances on Multicomponent Reactions Based on the Transamination Process of Electron Deficient Enamines[J]. Chinese Journal of Organic Chemistry, 2014 , 34(5) : 876 -885 . DOI: 10.6023/cjoc201312016
[1] Mukherjee, S.; Yang, J. W.; Hoffmann, S.; List, B. Chem. Rev. 2007, 107, 5471.
[2] Grondal, C.; Jeanty, M.; Enders, D. Nat. Chem. 2010, 3, 167.
[3] Kuehne, M. E. J. Am. Chem. Soc. 1959, 81, 5400.
[4] Malhotra, S. K.; Hostynek, J. J.; Lundin, A. F. J. Am. Chem. Soc. 1968, 90, 6565.
[5] Hayakawa, Y.; Yokoyama, K.; Noyori, R. J. Am. Chem. Soc. 1978, 100, 1799.
[6] Koradin, C.; Gommermann, N.; Polborn, K.; Knochel, P. Chem. Eur. J. 2003, 9, 2797.
[7] Koradin, C.; Polborn, K.; Knochel, P. Angew. Chem., Int. Ed. 2002, 41, 2535.
[8] Enders, D.; Huttl, M. R. M.; Grondal, C.; Raabe, G. Nature 2006, 441, 861.
[9] List, B. Acc. Chem. Res. 2004, 37, 548.
[10] Han, J.; Paton, R. S.; Xu, B.; Hammond, G. B. Synthesis 2013, 463.
[11] Xu, D.-Q.; Xia, H.-B.; Luo, S.-P.; Tang, J.; Zhang, S.; Jiang, J.-R.; Xu, Z.-Y. Angew. Chem., Int. Ed. 2009, 48, 3821.
[12] Sulzer-Mossé, S.; Alexakis, A. Chem. Commun. 2007, 3123.
[13] Han, Y.; Sun, J.; Sun, Y.; Gao, H.; Yan, C. G. Chin. J. Org. Chem. 2012, 32, 1557 (in Chinese).
(韩莹, 孙晶, 孙岩, 高红, 颜朝国, 有机化学, 2012, 32, 1557.)
[14] Li, M.; Guo, W. S.; Wen, L. R.; Yang, H. Z. Chin. J. Org. Chem. 2006, 26, 1192 (in Chinese).
(李明, 郭维斯, 文丽荣, 杨华铮, 有机化学, 2006, 26, 1192.)
[15] Wang, Y.-F.; Izawa, T.; Kobayashi, S.; Ohno, M. J. Am. Chem. Soc. 1982, 104, 6465.
[16] Neumann, J. J.; Suri, M.; Glorius, F. Angew. Chem., Int. Ed. 2010, 49, 7790.
[17] Refvik, M. D.; Schwan, A. L. J. Org. Chem. 1996, 61, 4232.
[18] Yang, Q.-Z.; Siri, O.; Braunstein, P. Chem. Commun. 2005, 2660.
[19] Lorber, C.; Vendier, L. Dalton Trans. 2013, 42, 12203.
[20] Gupta, S.; Agarwal, P. K.; Kundu, B. Tetrahedron Lett. 2010, 51, 1887.
[21] Liu, Y. Y.; Zhou, R. H.; Wan, J.-P. Synth. Commun. 2013, 43, 2475.
[22] Al-Saleh, El-Apasery, M. A.; Abdel-Aziz, R. S.; Elnagdi, M. H. J. Heterocycl. Chem. 2005, 42, 563.
[23] Zhou, Z.-Z.; Liu, F.-S.; Shen, D.-S.; Tan, C.; Luo, L.-Y. Inorg. Chem. Commun. 2011, 14, 659.
[24] Al-Saleh, B.; Abdelkhalik, M. M.; Eltoukhy, A. M.; Elnagdi, H. J. Heterocycl. Chem. 2002, 39, 1035.
[25] Reddy, G. J.; Latha, D.; Thirupathaiah, C.; Rao, K. S. Tetrahedron Lett. 2005, 46, 301.
[26] Kantevari, S.; Chary, M. V.; Vuppalapati, S. V. N. Tetrahedron 2007, 63, 13024.
[27] Kantevary, S.; Chary, M. V.; Vuppalapati, S. V. N.; Lingaiah, N. J. Heterocycl. Chem. 2008, 45, 1099.
[28] Kantevary, S.; Patpi, S. R.; Addla, D.; Putapatri, S. R.; Sridhar, B.; Yogeeswari, P.; Sriram, D. ACS Comb. Sci. 2011, 13, 427.
[29] Kantevary, S.; Addla, D.; Sridhar, B. Synthesis 2010, 3745.
[30] Kantevary, S.; Putapatri, S. R. Synlett 2010, 2251.
[31] Siddiqui, Z. N.; Ahmed, N.; Farooq, F.; Khan, K. Tetrahedron Lett. 2013, 54, 3599.
[32] Wan, J.-P.; Gan, S.-F.; Sun, G.-L.; Pan, Y.-J. J. Org. Chem. 2009, 74, 2862.
[33] Wan, J.-P.; Wang, C. P.; Pan, Y. J. Tetrahedron 2011, 67, 922.
[34] Muthusaravanan, S.; Perumal, S.; Almansour, A. I. Tetrahedron Lett. 2012, 53, 1144.
[35] Wan, J.-P.; Zhou, R. H.; Liu, Y. Y.; Cai, M. Z. RSC Adv. 2013, 3, 2477.
[36] Muthusaravanan, S.; Devi bala, B.; Perumal, S. Tetrahedron Lett. 2013, 54, 5302.
[37] Muthusaravanan, S.; Sasikumar, C.; Devi bala, B.; Perumal, S. Green Chem. 2014, 16, 1297.
[38] Petrow, V. A. J. Chem. Soc. 1946, 888.
[39] Mirza-Aghayan, M.; Langrodi, M. K.; Rahimifard, M.; Boukherroub, R. Appl. Organomet. Chem. 2009, 23, 267.
[40] Yang, J. Y.; Wang, C. Y.; Xie, X.; Li, H.-F.; Li, Y. Z. Eur. J. Org. Chem. 2010, 4189.
[41] Liu, Z. M.; Zhang, L. L.; Sun, J.; Yan, C. G. Chin. J. Chem. 2013, 31, 479.
[42] Sirijindalert, T.; Hansuthirakul, K.; Rashatasakhon, P.; Sukwattanasinitt, M.; Ajavakom, A. Tetrahedron 2010, 66, 5161.
[43] Sasada, T.; Kobayashi, F.; Moriuchi, M.; Sakai, N.; Konakahara, T. Synlett 2011, 2029.
[44] Wan, J.-P.; Liu, Y. Y. Synthesis 2010, 3943.
[45] Wan, J.-P.; Pan, Y.-J. Chem. Commun. 2009, 2768.
[46] Darwish, E. S.; Abdelhamid, I. A.; Nasra, M. A.; Abdel-Gallil, F. M.; Fleita, D. H. Helv. Chim. Acta 2010, 93, 1204.
[47] Chen, Z. G.; Wang, D.; Li, Y. N.; Wang, Y. J.; Hu, J. L.; Xia, W. Acta Chim. Sinica 2012, 70, 2236 (in Chinese).
(陈战国, 王丹, 李亚男, 王英杰, 胡均利, 夏伟, 化学学报, 2012, 70, 2236.)
/
| 〈 |
|
〉 |