

Chinese Journal of Organic Chemistry >
Novel and Efficient Synthesis of 6-Bromofuro[2,3-d]pyrimidine Bicyclic Nucleosides
Received date: 2013-11-13
Revised date: 2013-12-28
Online published: 2014-01-22
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21172057, 21272058), the Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT) (No. IRT 1061), and the Research Fund for the Doctoral Program of Higher Education (RFDP) (No. 20114104110005).
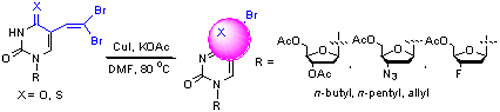
6-Bromofuro[2,3-d]pyrimidine bicyclic nucleosides are the key intermediates for the preparation of novel furo[2,3-d]pyrimidine bicyclic nucleoside derivatives with remarkable antiviral potency. The literature protocol for their synthesis involves a multi-step procedure and palladium catalyst. In this paper, an economical and practical method for their preparation is developed via condensation of the easily obtainable 5-formyl pyrimidine nucleosides with carbon tetrabromide and subsequent cyclization promoted by copper iodide.
Fan Xuesen , Li Peiyuan , Li Kun , Zhang Xinying , Zhao Wan . Novel and Efficient Synthesis of 6-Bromofuro[2,3-d]pyrimidine Bicyclic Nucleosides[J]. Chinese Journal of Organic Chemistry, 2014 , 34(5) : 999 -1005 . DOI: 10.6023/cjoc201311020
[1] (a) De Clercq, E. Rev. Med. Virol. 2009, 19, 287.
(b) De Clercq, E. J. Med. Chem. 2010, 53, 1438.
[2] Zhang, X.-Y.; Wang, Y.-Y.; Feng, D.; Fan, X.-S. Chin. J. Org. Chem. 2010, 30, 797 (in Chinese).
(张新迎, 王洋洋, 冯东, 范学森, 有机化学, 2010, 30, 797.)
[3] Topalis, D.; Pradère, U.; Roy, V.; Caillat, C.; Azzouzi, A.; Broggi, J.; Snoeck, R.; Andrei, G.; Lin, J.; Eriksson, S.; Alexandre, J. A. C.; El-Amri, C.; Deville-Bonne, D.; Meyer, P.; Balzarini, J.; Agrofoglio, L. A. J. Med. Chem. 2011, 54, 222.
[4] Srivastav, N. C.; Mak, M.; Agrawal, B.; Tyrrell, D. L. J.; Kumar, R. Bioorg. Med. Chem. Lett. 2010, 20, 6790.
[5] McGuigan, C.; Yarnold, C. J.; Jones, C.; Velázquez, S.; Barucki, H.; Brancale, A.; Andrei, G.; Snoeck, R.; De Clercq, E.; Balzarini, J. J. Med. Chem. 1999, 42, 4479.
[6] Balzarini, J.; McGuigan, C. J. Antimicrob. Chemother. 2002, 50, 5.
[7] Janeba, Z.; Balzarini, J.; Andrei, G.; Snoeck, R.; De Clercq, E.; Robins, M. J. J. Med. Chem. 2005, 48, 4690.
[8] Januszczyk, P.; Fogt, J.; Boryski, J.; Izawa, K.; Onishi, T.; Neyts, J.; De Clercq, E. Nucleosides Nucleotides Nucleic Acids 2009, 28, 713.
[9] Kifli, N.; De Clercq, E.; Balzarini, J.; Simons, C. Bioorg. Med. Chem. 2004, 12, 4245.
[10] Robins, M. J.; Barr, P. J. J. Org. Chem. 1983, 48, 1854.
[11] Jin, Y.-Y.; Xiao, Q.; Ju, Y. Chin. J. Org. Chem. 2009, 29, 44 (in Chinese).
(靳玄烨, 肖强, 巨勇, 有机化学, 2009, 29, 44.)
[12] McGuigan, C.; Barucki, H.; Blewett, S.; Carangio, A.; Andrei, G.; Snoeck, R.; De Clercq, E.; Balzarini, J. J. Med. Chem. 2000, 43, 4993.
[13] De Clercq, E. Med. Res. Rev. 2009, 29, 571.
[14] Migliore, M. Antiviral Chem. Chemother. 2010, 20, 107.
[15] Robins, M. J.; Miranda, K.; Rajwanshi, V. K.; Peterson, M. A.; Andrei, G.; Snoeck, R.; De Clercq, E.; Balzarini, J. J. Med. Chem. 2006, 49, 391.
[16] Newman, S. G.; Aureggi, V.; Bryan, C. S.; Lautens, M. Chem. Commun. 2009, 5236.
[17] Chen, W.; Zhang, Y.-C.; Zhang, L.; Wang, M.; Wang, L. Chem. Commun. 2011, 10476.
[18] Fan, X.-S.; Wang, Y.-Y.; Qu, Y.-Y.; Xu, H.-Y.; He, Y.; Zhang, X.-Y.; Wang, J.-J. J. Org. Chem. 2011, 76, 982.
[19] Desai, N. B.; McKelvie, N.; Ramirez, F. J. Am. Chem. Soc. 1962, 84, 1745.Corey, E. J.; Fuchs, P. L. Tetrahedron Lett. 1972, 13, 3769.
/
| 〈 |
|
〉 |