

Chinese Journal of Organic Chemistry >
A Facile and Efficient Synthesis of 2-Amino-1,3-dicarbonitrile-4-aryl-5,6,7,8,9,10,11,12,13,14-decahydrobenzo[12]annulene Derivatives
Received date: 2013-11-21
Revised date: 2014-01-10
Online published: 2014-01-22
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 20936007, 51134021, 51074153, 21276268), the National Basic Research Program of China (Nos. 2011CB201302, 2012CB215302).
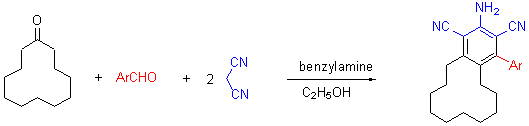
A facile and efficient synthesis of 2-amino-1,3-dicarbonitrile-4-aryl-5,6,7,8,9,10,11,12,13,14-decahydrobenzo[12]-annulene derivatives by the reaction of aromatic aldehydes, cyclododecanone, and malononitrile in the presence of benzylamine in 95% EtOH is reported. Benzyamine as base to catalyze this kind of reaction has not been reported. This protocol has the advantages of short reaction time, mild conditions, and easy work-up. The products were identified by IR, 1H NMR and HRMS. The reported method is the efficient approach for the synthesis of these compounds.
Key words: annulene; cyclododecanone; malononitrile; multicomponent reactions
Xie Ruilun , Zong Zhimin , Wei Xianyong , Gao Yanian , Cheng Ming , Rong Liangce . A Facile and Efficient Synthesis of 2-Amino-1,3-dicarbonitrile-4-aryl-5,6,7,8,9,10,11,12,13,14-decahydrobenzo[12]annulene Derivatives[J]. Chinese Journal of Organic Chemistry, 2014 , 34(5) : 1006 -1009 . DOI: 10.6023/cjoc201311036
[1] Murata, H.; Ishitani, H.; Iwamoto, M. Org. Biomol. Chem. 2010, 8, 1202.
[2] Moafi, L.; Ahadi, S.; Khavasi, H. S.; Bazgir, A. Synthesis 2011, 1399.
[3] Wang, H. Y.; Zou, Y.; Tao, C. Z. Chin. J. Org. Chem 2011, 31, 2161 (in Chinese).
(王慧彦, 邹毅, 陶传洲, 有机化学, 2011, 31, 2161.)
[4] Dumur, F.; Gautier, N.; Gallego-Planas, N.; Sahin, Y.; Levillain, E.; Mercier, N.; Hudhomme, P. J. Org. Chem. 2004, 69, 2164.
[5] Xiao, Y.; Qian, X. H. Tetrahedron Lett. 2003, 44, 2087.
[6] Sepiol, J.; Milart, P. Tetrahedron 1985, 41, 5261.
[7] Griffiths, J.; Lockwood, M.; Roozpeikar, B. J. Chem. Soc., Perkin Trans. 1 1977, 1608.
[8] Rong, L. C.; Han, H. X.; Jiang, H.; Tu, S. J. Synth. Commun. 2008, 38, 3530.
[9] Rong, L. C.; Tao, S. M.; Xia, S.; Liu, L. H.; Yin, S.; Shi, Y. H. Res. Chem. Intermed. 2012, 38, 1647.
[10] Rong, L. C.; Gao, L. J.; Han, H. X.; Jiang, H.; Dai, Y. S.; Tu, S. J. Synth. Commun. 2010, 40, 289.
[11] Wang, X. S.; Zhang, M. M.; Li, Q.; Yao, C. S.; Tu, S. J. Tetrahedron 2007, 63, 5265.
[12] Rong, L. C.; Han, H. X.; Jiang, H.; Tu, S. J. Synth. Commun. 2009, 9, 3493.
[13] Cui, S. L.; Lin, X. F.; Wang, Y. G. J. Org. Chem. 2005, 70, 2866.
[14] Shaterian, H. R.; Honarmand, M.; Oveisi, A. R. Monatsh. Chem. 2010, 141, 557.
[15] Adib, M.; Mohammadi, B.; Ansari, S.; Bijanzadeh, H. R.; Zhu, L. Synthesis 2010, 1526.
[16] Subhash, B.; Alissa, H.; Hari, K.; Grigoriy, S. Tetrahedron Lett. 2011, 52, 1878.
[17] Yi, C.; Blum, C.; Liu, S.; Frei, G.; Neels, A.; Renaud, P.; Leutwyler, S.; Decurtins, S. J. Org. Chem. 2008, 73, 3596.
[18] Xin, X.; Wang, Y.; Xu, W.; Lin, Y.; Duan, H.; Dong, D. Green Chem. 2010, 12, 893.
/
| 〈 |
|
〉 |