

Chinese Journal of Organic Chemistry >
Tin-Mediated “One-Pot” Synthesis of Homoallylhydrazides from Aldehydes, Aryl Acylhydrazines and Allyl Bromide
Received date: 2013-11-26
Revised date: 2014-01-09
Online published: 2014-01-22
Supported by
Project supported by the National Natural Science Foundation of China (No. 21062016).
An one-pot reaction was developed for the synthesis of homoallylhydrazides by treating aldehydes, aryl acylhydrazines and allyl bromide with tin powder at room temperature in tetrahydrofuran. The reaction proceeded smoothly without using any catalyst under mild conditions to give the corresponding products in high yields.
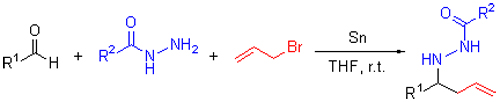
Key words: one pot; tin powder; aldehydes; acylhydrazines; homoallylhydrazides
Lu Ailing , Wang Fengjiao , Huang Danfeng , Wang Kehu , Su Yingpeng , X? Yanli , Hu Yulai . Tin-Mediated “One-Pot” Synthesis of Homoallylhydrazides from Aldehydes, Aryl Acylhydrazines and Allyl Bromide[J]. Chinese Journal of Organic Chemistry, 2014 , 34(5) : 948 -955 . DOI: 10.6023/cjoc201311042
[1] (a) Piao, F.; Mishra, M. K.; Jang, D. O. Tetrahedron 2012, 68, 7050.
(b) Morgen, M.; Bretzke, Z.; Menche, P.; Li, D. Org. Lett. 2010, 12, 4494.
(c) Airiau, E.; Girard, N.; Pizzeti, M.; Salvadori, J.; Taddei, M.; Mann, A. J. Org. Chem. 2010, 75, 8670.
(d) Denhez, C.; Vasse, J.- L.; Harakat, D.; Szymoniak, J. Tetrahedron: Asymmetry 2007, 18, 424.
(e) Kropf, J. E.; Meigh, I. C.; Bebbington, M. W. P.; Weinreb, S. M. J. Org. Chem. 2006, 71, 2046.
[2] (a) Yus, M.; Gonzalez-Gomez, J. C.; Foubelo, F. Chem. Rev. 2013, 113, 5595.
(b) Pandey, M. K.; Bisai, A.; Pandey, A.; Singh, V. K. Tetrahedron Lett. 2005, 46, 5039.
(c) Schmidt, A. M.; Eilbracht, P. J. Org. Chem. 2005, 70, 5528.
[3] For reviews: (a) Allvaro, G.; Savoia, D. Synlett 2002, 651.
(b) Kobayashi, S.; Ishitani, H. Chem. Rev. 1999, 99, 1069.
(c) Bloch, R. Chem. Rev. 1998, 98, 1407.
(d) Enders, D.; Reinhold, U. Tetrahedron: Asymmetry 1997, 8, 1895.
(e) Denmark, S. E.; Nicaise, O. J.-C. Chem. Commun. 1996, 999.
[4] For reviews: (a) Shen, Z.-L.; Wang, S.-Y.; Chok, Y.-K.; Xu, Y.-L.; Loh, T.-P. Chem. Rev. 2013, 113, 271.
(b) Kobayashi, S.; Mori, Y.; Fossey, J. S.; Salter, M. M. Chem. Rev. 2011, 111, 2626.
(c) Yus, M.; Foubelo, F. Chem. Rev. 2011, 111, 7774.
[5] For reviews of multicomponent reactions: (a) Dömling, A.; Wang, W.; Wang, K. Chem. Rev. 2012, 112, 3083.
(b) Candeias, N. R.; Montalbano, F.; Cal, P. M. S. D.; Gois, P. M. P. Chem. Rev. 2010, 110, 6169.
(c) von Wangelin, A. J.; Neumann, H.; Gördes, D.; Klaus, S.; Strübing, D.; Beller, M. Chem. Eur. J. 2003, 9, 4286.
(d) Dömling, A.; Ugi, I. Angew. Chem., Int. Ed. 2000, 39, 3168.
[6] (a) Roy, U. K.; Roy, S. Chem. Rev. 2010, 110, 2472.
(b) Davies, A. G.; Gielen, M.; Pannell, K. H.; Tiekink, E. R. T. Tin Chemistry: Fundamentals, Frontiers, and Applications, John Wiley & Sons, Chichester, U. K., 2008.
(c) Pereyre, M.; Quintard, J.-P.; Rahm, A. Tin in Organic Synthesis, Butterworths, London, 1987.
[7] (a) Narsaiah, A. V.; Kumar, J. K.; Narsimha, P. Synthesis 2010, 1609.
(b) Li, X.; Liu, X. H.; Fu, Y. Z.; Wang, L. J.; Zhou, L.; Feng, X. M. Chem. Eur. J. 2008, 14, 4796.
(c) Thirupathi, P.; Kim, S. S. Tetrahedron 2009, 65, 5168.
(d) Das, B.; Laxminarayana, K.; Ravikanth, B.; Ramarao, B. Tetrahedron Lett. 2006, 47, 9103.
[8] (a) Lin, M. H.; Lin, W. C.; Liu, H. J.; Chang, T. H. J. Org. Chem. 2013, 78, 1278.
(b) Lin, M. H.; Hung, S. F.; Lin, L. Z.; Tsai, W. S.; Chang, T. H. Org. Lett. 2011, 13, 332.
(c) Chan, T. H.; Yang, Y.; Li, C. J. J. Org. Chem. 1999, 64, 4452.
[9] (a) Sugiura, M.; Kobayashi, K. Angew. Chem., Int. Ed. 2005, 44, 5176.
(b) Hirabayashi, R.; Ogawa, C.; Sugiura, M.; Kobayashi, S. J. Am. Chem. Soc. 2001, 123, 9493.
[10] Zha, Z. G.; Hui, A. L.; Zhou, Y. Q.; Miao, Q.; Wang, Z. Y.; Zhang, H. C. Org. Lett. 2005, 7, 1903.
[11] Chen, W. J.; Liao, D. H. Chem. World 2006, (5), 285 (in Chinese).
(陈文杰, 廖道华, 化学世界, 2006, (5), 285.)
[12] Kim, S. J.; Jang, D. O. J. Am. Chem. Soc. 2010, 132, 12168.
[13] Lee, B. S.; Jang. D. O. Eur. J. Org. Chem. 2013, 3123.
[14] Tan, K. L.; Jacobsen, E. N. Angew. Chem., Int. Ed. 2007, 46, 1315.
/
| 〈 |
|
〉 |