

Chinese Journal of Organic Chemistry >
Progress in Asymmetric Synthesis of Galanthamine-Type Alkaloids
Received date: 2013-12-02
Revised date: 2014-01-07
Online published: 2014-02-10
Supported by
Project supported by the National Natural Science Foundation of China (No. 21302061), the China Postdoctoral Science Foundation (Nos. 2013T60318, 2012M510130) and the Jilin Province Science and Technology Development Plan Item (No. 20140520084JH).
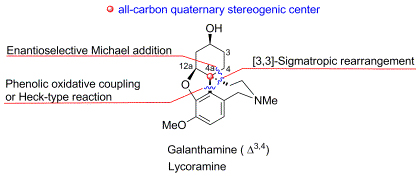
Galanthamine-type alkaloids are an important type of hydrodibenzofuran alkaloids. Their unique intriguing structures, significant biological activitives and pharmacological potential have attracted much attention of chemists and pharmacologists. The major challenge for their synthesis is how to construct the aryl-substituted all-carbon quaternary stereogenic center. In this paper, current strategies for asymmetric synthesis of galanthamine-type alkaloids are reviewed, and the different methods to prepare the quaternary carbon are discussed. The features of different strategies are also commented.
Wang Haiming , Chen Peng , Tang Meng . Progress in Asymmetric Synthesis of Galanthamine-Type Alkaloids[J]. Chinese Journal of Organic Chemistry, 2014 , 34(5) : 852 -864 . DOI: 10.6023/cjoc201312002
[1] Proskurnina, N. F.; Yakoleva, A. P. J. Gen. Chem. 1952, 22, 1899.
[2] (a) Han, S. Y.; Sweeney, J. E.; Bachman, E. S.; Schweiger, E. J.; Forloni, G.; Coyle, J. T.; Davis, B. M.; Joullie, M. M. Eur. J. Med. Chem. 1992, 27, 673。
(b) Marco-Contelles, J.; do Carmo Carreiras, M.; Rodriguez, C.; Villarroya, M.; Garcia, A. G. Chem. Rev. 2006, 106, 116.
[3] (a) Boers, G. M.; Kosley, R. W. Drugs Future 1996, 21, 621.
(b) Rainer, M. Drugs Today 1997, 33, 273.
(c) Mucke, H. A. M. Drugs Today 1997, 33, 259.
(d) Giacobini, E. Neurochem. Int. 1998, 32, 413.
(e) Weinstock, M. CNS Drugs 1999, 12, 307.
(f) Sramek, J. J.; Frackiewicz, E. J.; Cutler, N. R. Expert Opin. Investig. Drugs 2000, 9, 2393.
[4] Lilienfeld, S. CNS Drug Rev. 2002, 8, 159.
[5] Kondo, H.; Tomimura, K.; Ishiwata, S. J. Pharm. Soc. Jpn. 1932, 52, 433.
[6] (a) Fan, C. A.; Tu, Y. Q.; Song, Z. L.; Zhang, E.; Shi, L.; Wang, M.; Wang, B.; Zhang, S. Y. Org. Lett. 2004, 6, 4691.
(b) Hu, X. D.; Tu, Y. Q.; Zhang, E.; Gao, S.; Wang, S.; Wang, A.; Fan, C. A.; Wang, M. Org. Lett. 2006, 8, 1823.
[7] Chen, J. Q.; Xie, J. H.; Bao, D. H.; Liu, S.; Zhou, Q. L. Org. Lett. 2012, 14, 2714.
[8] Chen, P.; Bao, X.; Zhang, L. F.; Ding, M.; Han, X. J.; Li, J.; Zhang, G. B.; Tu, Y. Q.; Fan, C. A. Angew. Chem. Int. Ed. 2011, 50, 8161.
[9] (a) Parker, K. A.; Fokas, D. J. Am. Chem. Soc. 1992, 114, 9688.
(b) Hong, C. Y.; Kado, N.; Overman, L. E. J. Am. Chem. Soc. 1993, 115, 11028.
(c) Taber, D. F.; Neubert, T. D.; Rheingold, A. L. J. Am. Chem. Soc. 2002, 124, 12416.
(d) Stork, G.; Yamashita, A.; Adams, J.; Schulte, G. R.; Chesworth, R.; Miyazaki, Y.; Farmer, J. J. J. Am. Chem. Soc. 2009, 131, 11402.
(e) He, Z.-L.; Wu, Y.-L.; Hong, Y.-S.; Wu, Y.-K.; Yao, Z.-J. Total Synthesis of Natural Products—Alkaloids, Science Press, Beijing, 2009 (in Chinese).
(何子乐, 吴毓林, 洪永叁, 伍贻康, 姚祝军, 天然产物全合成荟萃——生物碱, 科学出版社, 北京, 2009.)
[10] (a) Gong, J.; Lin, G.; Sun, W.; Li, C. C.; Yang, Z. J. Am. Chem. Soc. 2010, 132, 16745.
(b) Peng, F.; Danishefsky, S. J. J. Am. Chem. Soc. 2012, 134, 18860.
(c) Lu, P.; Gu, Z.; Zakarian, A. J. Am. Chem. Soc. 2013, 135, 14552.
[11] Cha, J. Y.; Yeoman, J. T.; Reisman, S. E. J. Am. Chem. Soc. 2011, 133, 14964.
[12] (a) Marco-Contelles, J.; Perez-Mayoral, E.; van Nhien, A. N.; Postel, D. Targets Heterocycl. Syst. 2007, 11, 365.
(b) Fang, L.; Gou, S. H.; Zhang, Y. H. Chin. J. Org. Chem. 2011, 31, 286 (in Chinese).
(房雷, 苟少华, 张奕华, 有机化学, 2011, 31, 286.)
[13] Shieh, W.-C.; Carlson, J. A. J. Org. Chem. 1994, 59, 5463.
[14] Magnus, P.; Sane, N.; Fauber, B. P.; Lynch, V. J. Am. Chem. Soc. 2009, 131, 16045.
[15] (a) Kametani, T.; Sivaraman Premila, M.; Fukumoto, K. Heterocycles 1976, 4, 1111.
(b) Szewczyk, J.; Wilson, J. W.; Lewin, A. H.; Carroll, F. I. J. Heterocycl. Chem. 1995, 32, 195.
(c) Chaplin, D. A.; Johnson, N. B.; Paul, J. M.; Potter, G. A. Tetrahedron Lett. 1998, 39, 6777.
[16] (a) Barton, D. H. R.; Kirby, G. W. J. Chem. Soc. 1962, 806.
(b) Barton, D. H. R.; Kirby, G. W.; Taylor, J. B.; Thomas, G. M. J. Chem. Soc. 1963, 4545.
(c) Fuganti, C. Chim. Ind. (Milan) 1969, 51, 1254.
(d) Bhandarkar, J. G.; Kirby, G. W. J. Chem. Soc. (C) 1970, 1224.
(e) Eichhorn, J.; Takada, T.; Kita, Y.; Zenk, M. H. Phytochemistry 1998, 49, 1037.
[17] (a) Tomioka, K.; Shimizu, K.; Yamada, S.-I.; Koga, K. Heterocycles 1977, 6, 1752.
(b) Shimizu, K.; Tomioka, K.; Yamada, S.-I.; Koga, K. Heterocycles 1977, 8, 277.
(c) Shimizu, K.; Tomioka, K.; Yamada, S.-I.; Koga, K. Chem. Pharm. Bull. 1978, 26, 3765.
[18] (a) Kodama, S.; Hamashima, Y.; Nishide, K.; Node, M. Angew. Chem., Int. Ed. 2004, 43, 2659.
(b) Node, M.; Kodama, S.; Hamashima, Y.; Katoh, T.; Nishide, K.; Kajimoto, T. Chem. Pharm. Bull. 2006, 54, 1662.
[19] Trost, B. M.; Toste, F. D. J. Am. Chem. Soc. 2000, 122, 11262.
[20] (a) Trost, B. M.; Tang, W. Angew. Chem., Int. Ed. 2002, 41, 2795.
(b) Trost, B. M.; Tang, W.; Toste, F. D. J. Am. Chem. Soc. 2005, 127, 14785.
[21] Zang, Y.; Ojima, I. J. Org. Chem. 2013, 78, 4013.
[22] Satcharoen, V.; McLean, N. J.; Kemp, S. C.; Camp, N. P.; Brown, R. C. Org. Lett. 2007, 9, 1867.
[23] Kim, Y.-J.; Tae, J. Synlett 2006, 61.
[24] Choi, J.; Kim, H.; Park, S.; Tae, J. Synlett 2013, 379.
[25] Malachowski, W. P.; Paul, T.; Phounsavath, S. J. Org. Chem. 2007, 72, 6792.
[26] Tanimoto, H.; Kato, T.; Chida, N. Tetrahedron Lett. 2007, 48, 6267.
[27] Imuta, S.; Tanimoto, H.; Momose, M. K.; Chida, N. Tetrahedron 2006, 62, 6926.
[28] Kato, T.; Tanimoto, H.; Yamada, H.; Chida, N. Heterocycles 2010, 82, 563.
/
| 〈 |
|
〉 |