

Chinese Journal of Organic Chemistry >
A Selective O- and N-Acylation Protocol for Carbamyl Chloride
Received date: 2013-11-20
Revised date: 2014-01-15
Online published: 2014-02-14
Supported by
Project supported by the Guangzhou International Science and Technology Cooperation Projects (No. 11S52070024).
A practical and efficient selective O- and N-acylation protocol for carbamyl chloride by using different bases was developed. The structures of the products were identified by 1H NMR, 13H NMR, ESI-MS and HRMS. The mechanism for the selective acylation is proposed.
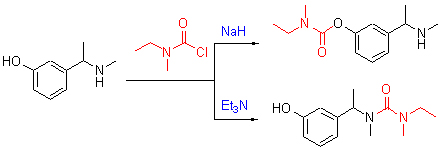
Key words: carbamyl chloride; acylation; selectivity; synthetic method
Lang Ming , Yan Ruizuo , Wang Yuqiang , Yu Pei . A Selective O- and N-Acylation Protocol for Carbamyl Chloride[J]. Chinese Journal of Organic Chemistry, 2014 , 34(6) : 1227 -1230 . DOI: 10.6023/cjoc201311035
[1] Wen, R. Organic Reactions for Drug Synthesis, 3rd ed., Chemical Industry Press, Beijing, 2010, p. 87 (in Chinese).
(闻韧, 药物合成反应第三版, 化学工业出版社, 北京, 2010, p. 87.)
[2] Gibson, H. W.; Ge, Z.; Jones, J. W.; Harich, K.; Pederson, A.; Dorn, H. C. J. Polym. Sci. A, Polym. Chem. 2009, 47, 6472.
[3] Fukuyama, T.; Liu, G. J. Am. Chem. Soc. 1996, 118, 7426.
[4] Chak, C. P.; Xuan, S.; Mendes, P. M.; Yu, J. C.; Cheng, C. H. Leung, K. C. ACS Nano 2009, 3, 2129.
[5] Cho, A.; Zhang, L.; Xu, J.; Lee, R.; Butler, T.; Metobo, S.; Aktoudianakis, V.; Lew, W.; Ye, H.; Clarke, M.; Doerffler, E.; Byun, D.; Wang, T.; Babusis, D.; Carey, A. C.; German, P.; Sauer, D.; Zhong, W.; Rossi, S.; Fenaux, M.; McHutchison, J. G.; Perry, J.; Feng, J.; Ray, A. S.; Kim, C. U. J. Med. Chem. 2013, 57, 1812.
[6] Pignataro, L.; Bovio, C.; Civera, M.; Piarulli, U.; Gennari, C. Chem.-Eur. J. 2012, 18, 10368.
[7] Mautino, M. R.; Kumar, S.; Jaipuri, F.; Waldo, J.; Kesharwani, T.; Zhang, X. WO 2011056652, 2011 [Chem. Abstr. 2011, 588962].
[8] Zhang, E.; Li, C.; Zhang, B. Y.; Lü, A. Q.; Fang, Y.; Feng, S. Q.; Liu, H. M. Chin. J. Org. Chem. 2013, 33, 1100 (in Chinese).
(张恩, 李聪, 张保寅, 吕爱桥, 方园, 冯思琦, 刘宏民, 有机化学, 2013, 33, 1100.)
[9] Youdim, M. B. Exp. Neurobiol. 2013, 22, 1.
[10] Sterling, J.; Herzig, Y.; Goren, T; Finkelstein, N.; Lerner, D.; Goldenberg, W.; Miskolczi, I.; Molnar, S.; Rantal, F.; Tamas, T.; Toth, G.; Zagyva, A.; Zekany, A.; Finberg, J.; Lavian, G.; Gross, A.; Friedman, R.; Razin, M.; Huang, W.; Krais, B.; Chorev, M.; Youdim, M. B.; Weinstock, M. J. Med. Chem. 2002, 45, 5260
[11] Gorske, B. C.; Stringer, J. R.; Bastian, B. L.; Fowler, S. A.; Blackwell, H. E. J. Am. Chem. Soc. 2009, 131, 16555.
/
| 〈 |
|
〉 |