

Chinese Journal of Organic Chemistry >
Synthesis and Application of 1-(Pyridin-2-yl)-2-azabuta-1, 3-dienes
Received date: 2013-12-19
Revised date: 2014-02-06
Online published: 2014-02-21
1-(Pyridin-2-yl)-2-azabuta-1,3-dienes (2) prepared from pyridine-2-carboxaldehyde underwent Diels-Alder reaction with electron-poor dienophiles such as alkenes and alkynes to give 6-(pyridin-2-yl)piperidin-2-ones and dihydro-6-(pyri- din-2-yl)pyridin-2-ones. Dihydro-6-(pyridin-2-yl)pyridin-2-one was oxidized with 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) to afford 6-(pyridin-2-yl) pyridin-2-one. Treatment of 6-(pyridin-2-yl)pyridin-2-one with phosphorus oxychloride gave bipyridine derivative.
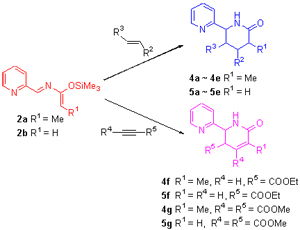
Key words: 2-azabuta-1,3-diene; Diels-Alder reaction; piperidin-2-one; pyridin-2-one
Yang Weifang , Xu Xiaoli , Zhang Zhanbin . Synthesis and Application of 1-(Pyridin-2-yl)-2-azabuta-1, 3-dienes[J]. Chinese Journal of Organic Chemistry, 2014 , 34(6) : 1161 -1166 . DOI: 10.6023/cjoc201312024
[1] (a) Liu, H.; Yan, X.; Chen, C.; Liu, Q.; Xi, C. Chem. Commun. 2013, 49, 5513.
(b) Palacios, F.; Heredia, I. P.; Rubiales, G. J. Org. Chem. 1995, 60, 2384.
(c) Mu, X.; Xia, Z.; Wang, C.; Panunzio, M.; Zeng, L. Chin. J. Org. Chem. 2009, 29, 61 (in Chinese).
(穆小静, 夏之宁, 王春燕, 有机化学, 2009, 29, 61.)
(d) Campos, P. J.; Caro, M.; Rodriguez, M. A. Tetrahedron 2013, 69, 7950.
(e) Watanabe, Y.; Washio, T.; Krishnamurthi, J.; Anada, M.; Hashimoto, S. Chem. Commun. 2012, 48, 6969.
(f) Novikov, M. S.; Smetanin, I. A.; Khlebnikov, A. F.; Rostovskii, N. V.; Yufit, D. S. Tetrahedron Lett. 2012, 53, 5777.
[2] (a) Jnoff, E.; Ghosez, L. J. Am. Chem. Soc. 1999, 121, 2617.
(b) Gouverneur, V.; Ghosez, L. Tetrahedron 1996, 52, 7585.
(c) Barluenga, J.; Tomas, M.; Ballesteros, A.; Santamaria, J.; Suarez-Sobrino, A. J. Org. Chem. 1997, 62, 9229.
(d) Panunzio, M.; Bandini, E.; D'Aurizio, A.; Xia, Z.; Mu, X. Synthesis 2008, 1753.
(e) Ntirampebura, D.; Ghosez, L. Synthesis 2002, 2043.
(f) Mathieu, B.; Ghosez, L. Tetrahedron 2002, 58, 8219.
[3] (a) Tang, Y. M.; Li, J.; Zhao, S. Y. Chin. J. Org. Chem. 2011, 31, 9 (in Chinese).
(唐玉敏, 李晶, 赵圣印, 有机化学, 2011, 31, 9.)
(b) Zhao, S. Y.; Huang, J.; Cheng, J.; Liu, B. S.; Chen, C. Chin. J. Org. Chem. 2012, 32, 651 (in Chinese).
(赵圣印, 黄婧, 程健, 刘宝硕, 陈晨, 有机化学, 2012, 32, 651.)
(c) Rong, L. C.; Liu, L. H.; Yin, S.; Xia, Y.; Wei, X. Y.; Zong, Z. M. Chin. J. Org. Chem. 2012, 32, 400 (in Chinese).
(荣良策, 刘丽华, 殷姗, 夏盛, 魏贤勇, 宗志敏, 有机化学, 2012, 32, 400.)
[4] Ghosez, L.; Bayard, P.; Nshimyumukiza, P.; Gouverneur, V.; Sainte, F.; Beaudegnies, R.; Rivera, M.; Frisque-Hesbain, A. M.; Wynants, C. Tetrahedron 1995, 51, 11021.
[5] Ghosez, L.; Jnoff, E.; Bayard, P.; Sainte, F.; Beaudegnies, R. Tetrahedron 1999, 55, 3387.
[6] Alves, M. J.; Duraes, M. M.; Fortes, A. G. Tetrahedron 2004, 60, 6541.
[7] Mitchinson, A.; Blackaby, W. P.; Robert, S. B.; Carling, W.; Lewis, R. T. Tetrahedron Lett. 2006, 47, 2257.
[8] Mittelbach, M. Synthesis 1988, 479.
/
| 〈 |
|
〉 |