

Chinese Journal of Organic Chemistry >
Progress in the Synthesis of 2-Substituted Benzoxazoles Derivatives
Received date: 2014-01-19
Revised date: 2014-02-20
Online published: 2014-03-12
Supported by
Project supported by the Natural Science Foundation of Hebei Province (No. B2013408014) and the Key Foundation of Langfang Teachers University (No. LSZZ201302).
2-Substituted benzoxazole derivatives play important roles in pharmaceuticals, pesticide and coordination catalysis, so the synthetic methods for 2-substituted benzoxazoles have been attracted widely. Recently, a series of efficient green methods such as microwave irradiation, water as solvent, solvent-free, combinatorial chemistry and transition metal catalysis etc. have been used to synthesize 2-substituted benzoxazoles derivatives. Based on different starting materials and different methods, the recent advances in synthesis of 2-substituted benzoxazole are reviewed.
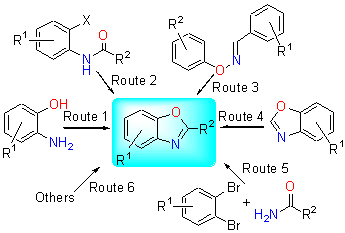
Xiao Liwei , Gao Hongjie , Kong Jie , Liu Guangxian , Peng Xiaoxia , Wang Shujun . Progress in the Synthesis of 2-Substituted Benzoxazoles Derivatives[J]. Chinese Journal of Organic Chemistry, 2014 , 34(6) : 1048 -1060 . DOI: 10.6023/cjoc201401030
[1] Razavi, H.; Palaninathan, S. K.; Powers, E. T. Angew. Chem., Int. Ed. 2003, 42, 2758.
[2] (a) Gao, R.; Zhang, M.; Liang, T.; Wang, F.; Sun, W.-H. Organometallics 2008, 27, 5641.
(b) Gao, R.; Li, Y.; Wang, F.; Sun, W.-H. Eur. J. Inorg. Chem. 2009, 4149.
[3] Ge, F.; Wang, Z.; Wan, W.; Lu, W.; Hao, J. Tetrahedron Lett. 2007, 48, 3251.
[4] Zhang, H. Z.; Zhou, C. H.; Geng, R. X.; Ji, Q. G. Chin. J. Org. Chem. 2011, 31, 1963 (in Chinese).
(张慧珍, 周成合, 耿蓉霞, 吉庆刚, 有机化学, 2011, 31, 1963.)
[5] Kawashita, Y.; Nakamichi, N.; Kawabata, H.; Hayashi, M. Org. Lett. 2003, 5, 3713.
[6] Haneda, S.; Gan, Z. B.; Eda, K.; Hayashi, M. Organometallics 2007, 26, 6551.
[7] Xiao, L. W. Chin. J. Org. Chem. 2011, 31, 878 (in Chinese).
(肖立伟, 有机化学, 2011, 31, 878.)
[8] Cao, K.; Tu, Y. Q.; Zhang, F. M. Sci. China Chem. 2010, 53, 130.
[9] Inamdar, S. M.; More, V. K.; Mandal, S. K. Tetrahedron Lett. 2013, 54, 579.
[10] Shavaleev, N. M.; Scopelliti R.; Gumy, F.; Bunzli, J. G. Inorg. Chem. 2009, 48, 6178.
[11] Heravi, M. M.; Sadjadi S.; Oskooie, H. A.; Shoar, R. H.; Bamoharram, F. F. J. Chin. Chem. Soc. 2008, 55, 890.
[12] Kumar, A.; Maurya, R. A.; Ahmad, P. J. Comb. Chem. 2009, 11, 198.
[13] Firouz, M. M.; Ghasem, R. B.; Hossein, I. Synth. Commun. 2006, 36, 2543.
[14] Balaswamy, G.; Srinivas K.; Pradeep P.; Sarangapani M. Int. J. Chem. Sci. 2012, 10, 619.
[15] Ertan, T.; Yildiz, I.; Tekiner-Gulbas, B.; Bolelli, K.; Temiz-Arpaci, O.; Ozkan, S.; Kaynak, F.; Yalcin, I.; Aki. E. Eur. J. Med. Chem. 2008, 1.
[16] Wei, C. X.; Wu, D.; Sun, Z. G.; Chai, K. Y.; Quan, Z. S. Med. Chem. Res. 2010, 19, 925.
[17] Soares, A. M. S.; Costa, S. P. G.; Goncalves, M. S. T. Tetrahedron 2010, 66, 8189.
[18] Zhou, H. J.; Wang, L. G.; Yin, B. S. Chem. Res. 2003, 14, 39 (in Chinese).
(周红军, 王立格, 尹帮少, 化学研究, 2003, 14, 39.)
[19] Heravi, M. M.; Sadjadi, S.; Oskooie, H. A.; Shoar, R. H.; Bamoharram, F. F. J. Chin. Chem. Soc. 2008, 55, 890.
[20] Boyle, K. E.; MacMillan, K. S.; Ellis, D. A.; Lajiness, J. P.; Robertson, W. M.; Boger, D. L. Bioorg. Med. Chem. Lett. 2010, 20, 1854.
[21] Radi, M.; Saletti, S.; Botta, M. Tetrahedron Lett. 2008, 49, 4464.
[22] Xiao, L.-W.; Zhang, M.; Sun, W.-H. Chem. Res. Chin. Univ. 2010, 25, 1
[23] Mohammadpoor-Baltork, I.; Moghadam, M.; Tangestaninejad, S.; Mirkhani, V.; Zolfigol, M. A.; Hojati, S. F. J. Iran. Chem. Soc. 2008, 5, 65.
[24] Lim, H. J.; Myung, Do.; Lee, Y. C.; Jung M. H. J. Comb. Chem. 2008, 10, 501.
[25] Bastug, G.; Eviolitte, C.; Markó, I. E. Org. Lett. 2012, 14, 3502.
[26] Jeyanthi, P.; Pazhanisamy, P. Int. J. ChemTech Res. 2010, 2, 1170.
[27] Christina, P.; Stella, R.; Rajam, S.; Venkatraman, B. R. J. Chem. Pharm. Res. 2012, 4, 2988.
[28] Pottorf, R. S.; Chadha, N. K.; Katkevics, M.; Ozola, V.; Suna, E.; Ghane, H.; Regberg, T.; Player, M. R. Tetrahedron Lett. 2002, 44, 175.
[29] Shoar, R. H.; Heidary, M.; Farzaneh, M.; Malakouti, R. Synth. Commun. 2009, 39, 1742.
[30] Nadaf, R. N.; Siddiqui, S. A.; Lahoti, R. J. J. Mol. Catal. A: Chem. 2004, 214, 155.
[31] Chun, Y. S.; Lim, S. J.; Oh, S. J.; Moon, D. H.; Kim, D. J.; Cho, C. G.; Yoo, K. H. Bull. Korean Chem. Soc. 2008, 29, 1765.
[32] Gao, R.; Xiao, L.-W.; Hao, X.; Sun, W.-H.; Wang, F. S. Dalton Trans. 2008, 5645.
[33] Gu, L. J.; Jin, C.; Guo, J. M.; Zhang, L. Z.; Wang, W. Chem. Commun. 2013, 49, 10968.
[34] Wilfred, C. D.; Taylor, R. J. K. Synlett 2004, 1628.
[35] Wu, M.; Hu, X.; Liu, J.; Liao, Y.; Deng, G.-J. Org. Lett. 2012, 14, 2722.
[36] Endo, Y.; Bäckvall, J. E. Chem.-Eur. J. 2012, 43, 13609.
[37] Jin, X.; Liu, Y.; Lu, Q.; Yang, D.; Sun, J.; Qin, S.; Zhang, J.; Shen, J.; Chu, C.; Liu, R. Org. Biomol. Chem. 2013, 11, 3776.
[38] Nguyen, T. B.; Ermolenko, L.; Dean, W. A.; Al-Mourabit, A. Org. Lett. 2012, 14, 5948.
[39] Boissarie, P. J.; Hamilton, Z. E.; Lang, S.; Murphy, J. A.; Suckling, C. J. Org. Lett. 2011, 13, 6256.
[40] Zhang, C. R.; Wang, L.; Ge, Y. L.; Ju, X. L. Chin. J. Org. Chem. 2007, 27, 1432 (in Chinese).
(张成仁, 王柳, 葛燕丽, 巨修练, 有机化学, 2007, 27, 1432.)
[41] Bonnamour, J.; Bolm, C. Org. Lett. 2008, 10, 2665.
[42] Evindar, G.; Batey, R. A. J. Org. Chem. 2006, 71, 1802.
[43] Gereon, A.; Frank, G. A. Adv. Synth. Catal. 2004, 346, 1661.
[44] Saha, P.; Ramana, T.; Purkait, N.; Ali, M. A.; Paul, R.; Punniyamurthy, T. J. Org. Chem. 2009, 74, 8719.
[45] Saha, P.; Ali, M. A.; Ghosh, P.; Punniyamurthy, T. Org. Biomol. Chem. 2010, 8, 5692.
[46] Viirre, R. D.; Evindar, G.; Batey, R. A. J. Org. Chem. 2008, 73, 3452.
[47] Ueda, S.; Nagasawa, H. Angew. Chem., Int. Ed. 2008, 47, 6411.
[48] Sedelmeier, J.; Lima, F.; Litzler, A.; Martin, B.; Venturoni, F. Org. Lett. 2013, 15, 5546.
[49] Suresh, D.; Dhakshinamoorthy, A.; Pitchumani, K. Tetrahedron Lett. 2013, 54, 6415.
[50] Wu, G.; Zhou, J.; Zhang, M.; Hu, P.; Su, W. Chem. Commun. 2012, 48, 8964.
[51] Mao, Z.; Wang, Z.; Xu, Z.; Huang, F.; Yu, Z.; Wang, R. Org. Lett. 2012, 14, 3854.
[52] Yu, D.; Lu, L.; Shen, Q. Org. Lett. 2013, 15, 940.
[53] Zhang, W.; Zeng, Q.; Zhang, X.; Tian, Y.; Yue, Y.; Guo, Y.; Wang, Z. J. Org. Chem. 2011, 76, 4741.
[54] Gerelle, M.; Dalencon, A. J.; Willis, M. C. Tetrahedron Lett. 2012, 53, 1954.
[55] Ranjit, S.; Liu, X. G. Chem. Eur. J. 2011, 17, 1105.
[56] Xie, K.; Yang, Z.; Zhou, X.; Li, X. ; Wang, S.; Tan, Z.; An, X.; Guo, C. Org. Lett. 2010, 12, 1564.
[57] Inomata, H.; Ogata, K.; Fukuzawa, S.; Hou, Z. M. Org. Lett. 2012, 14, 3986.
[58] Teo, Y. C.; Riduan, S. N.; Zhang, Y. G. Green Chem. 2013, 15, 2365.
[59] Xu, D.; Wang, W.; Miao, C.; Zhang, Q.; Xia, C.; Sun, W. Green Chem. 2013, 15, 2975.
[60] Guo, S.; Qian, B.; Xie, Y.; Xia, C.; Huang, H. Org. Lett. 2011, 13, 522.
[61] Chen, S.; Zheng, K.; Chen, F. Tetrahedron Lett. 2012, 53, 6297.
[62] Inomata, H.; Toh, A.; Mitsui, T.; Fukuzawa, S. I. Tetrahedron Lett. 2013, 54, 4729.
[63] Viirre, R. D.; Evindar, G.; Batey, R. A. J. Org. Chem. 2008, 73, 3452.
[64] Wu, X.-F.; Neumann, H.; Neumann, S.; Beller, M. Tetrahedron Lett. 2013, 54, 3040.
[65] Gadakh, A. V.; Pandit, C.; Rindhe, S. S.; Karale, B. K. Bioorg. Med. Chem. Lett. 2010, 20, 5572.
[66] Guru, M. M.; Ali, M. A.; Punniyamurthy, T. Org. Lett. 2011, 13, 1194.
[67] Marsden, S. P.; McGonagle, A. E.; McKeever-Abbas, B. Org. Lett. 2008, 10, 2589.
[68] Okitsu, T.; Nagase, K.; Nishio, N.; Wada, A. Org. Lett. 2012, 14, 708.
[69] Chen, C.-Y.; Andreani, T.; Li, H. Org. Lett. 2011, 13, 63.
/
| 〈 |
|
〉 |