

Chinese Journal of Organic Chemistry >
Synthesis and Biological Evaluation of New Retinoid Derivatives
Received date: 2014-02-11
Revised date: 2014-03-31
Online published: 2014-04-01
Supported by
Project supported by the Innovation Program of Shanghai Municipal Education Commission (No. 13ZZ047), the Scientific Research Foundation for the Returned Overseas Chinese Scholars (No. 2011-1139) and the State Education Ministry and the Fundamental Research Funds for the Central Universities.
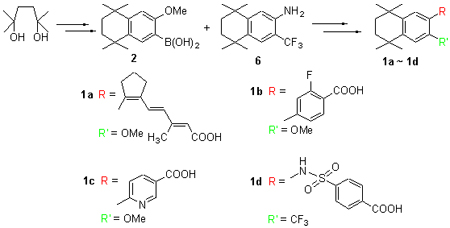
The key intermediate boronic acid 2 was synthesized via sequential reactions of Friedel-Crafts alkylation, bromination and nucleophilic reaction by using anisole as starting material. Hydroxyacetone was converted to bromide compound 3 by the Wittig reaction, bromination, Arbuzov reaction and Horner-Wadsworth-Emmons (HWE) reaction. Three novel retinoid derivatives 1a~1c can be obtained via the Suzuki coupling reaction of the boronic acid 2 with bromide compound 3, methyl 4-bromo-2-fluorobenzoate (4) or methyl 6-bromonicotinate (5) and followed by hydrolysis of the corresponding esters. In addition, trifluoromethyl substituted retinoid derivative 1d was synthesized via sequential reactions of Friedel-Crafts alkylation, nitration, trifluoromethylation, reduction and nucleophilic reaction with bromobenzene as strarting material. The structures of four new retinoid derivatives 1a~1d were confirmed by 1H NMR, 13C NMR, IR and HRMS analyses. Furthermore, the inducing differentiation abilities of molecules 1a~1d for HL-60 cells were tested, and the results revealed that these four retinoid derivatives are biological active. The IC50 values of fluorinated derivatives 1b and 1d are lower than that of commercial medicine all trans retinoid acid (ATRA).
Key words: retinoid derivative; synthesis; organofluorine compounds; antineoplastic
Xu Zhihan , Pan Shen , Huang Yangen . Synthesis and Biological Evaluation of New Retinoid Derivatives[J]. Chinese Journal of Organic Chemistry, 2014 , 34(7) : 1391 -1398 . DOI: 10.6023/cjoc201401047
[1] Barnard, J. H.; Collings, J. C.; Whiting, A.; Przyborski, S. A.; Marder, T. B. Chem. Eur. J. 2009, 15, 11430.
[2] Vaezi, M. F.; Alam, M.; Muccio, D. D.; Rogers, T. S.; Simpson-Herren, L.; Wille, J. J.; Hill, D. L.; Doran, T. I.; Brouillette, W. J.; Muccio, D. D. J. Med. Chem. 1994, 37, 4499.
[3] Alam, M.; Zhestkov, V.; Sani, B. P.; Venepally, P.; Levin, A. A.; Kazmer, S.; Li, E.; Norris, A. W.; Zhang, X. J. Med. Chem. 1995, 38, 2302.
[4] Qing, F. L.; Fan, J. F. Bioorg. Med. Chem. Lett. 1997, 7, 2117.
[5] Qing, F. L.; Fan, J. F. J. Fluorine Chem. 1999, 96, 159.
[6] Qing, F. L.; Yue, X. J. J. Chem. Soc., Perkin Trans. 1 1997, 3053.
[7] Boehm, M. F.; Zhang, L.; Badea, B. A.; White, S. K.; Mais, D. E.; Berger, E.; Suto, C. M.; Goldman, M. E.; Heyman, R. A. J. Med. Chem. 1994, 37, 2930.
[8] Faul, M. M.; Ratz, A. M.; Sullivan, K. A.; Trankle, W. G.; Winneroski, L. L. J. Org. Chem. 2001, 66, 5772.
[9] Farmer, L. J.; Jeong, S.; Kallel, E. A.; Koch, S. S. C.; Croston, G. E.; Flatten, K. S.; Heyman, R. A.; Nadzan A. M. Bioorg. Med. Chem. Lett. 1997, 7, 2393.
[10] Wagner, C. E.; Jurutka, P. W.; Marshall, P. A.; Groy, T. L.; Vaart, A.; Ziller, J. W.; Furmick, J. K.; Graeber, M. E.; Matro, E.; Miguel, B. V.; Tran, I. T.; Kwon, J.; Tedeschi, J. N.; Moosavi, S.; Danishyar, A.; Philp, J. S.; Khamees, R. O.; Jackson, J. N.; Grupe, D. K.; Badshah, S. L.; Hart, J. W. J. Med. Chem. 2009, 52, 5950.
[11] Liu, X. Y.; Li, Z. Z.; Wu, Y. D.; Zhang, Z. Y.; Liang, S. Z.; Jia, X. L.; Xiang, J. N. Acta Chim. Sinica 2008, 66, 1086 (in Chinese).
(刘西洋, 李治章, 吴运东, 张尊英, 梁盛宗, 贾晓雷, 向建南, 化学学报, 2008, 66, 1086.)
[12] Li, W. J.; Nelson, D. P.; Jensen, M. S.; Hoerrner, R. S.; Cai, D. W.; Larsen, R. D.; Reider, P. J. J. Org. Chem. 2002, 67, 5394.
[13] Magoulas, G. E.; Bariamis, S. E.; Athanassopoulos, C. M.; Haskopoulos, A.; Dedes, P. G.; Krokidis, M. G.; Karamanos, N. K.; Kletsas, D.; Papaioannou, D.; Maroulis, G. Eur. J. Med. Chem. 2011, 46, 721.
[14] Miyashita, K.; Sakai, T.; Imanishi, T. Org. Lett. 2003, 5, 2683.
[15] Qing, F.-L.; Qiu, X. Organofluorine Chemistry, Science Press, Beijing, 2007 (in Chinese).
(卿凤翎, 邱小龙, 有机氟化学, 科学出版社, 北京, 2007.)
[16] Chen, Q. Y.; Wu, S. W. J. Chem. Soc., Chem. Commun. 1989, 705.
[17] Zheng, X. X.; Oda, H.; Takamatsu, K.; Sugimoto, Y.; Tai, A.; Akaho, E.; Ali, H. I.; Oshiki, T.; Kakuta, H.; Sasaki, K. Biol. Med. Chem. 2007, 15, 1014.
[18] Hao, B. C.; Zhang, Y. H.; Lai, Y. S.; Yuan, S. T.; Zhang, L. Y. Chin. J. Org. Chem. 2007, 27, 629 (in Chinese).
(郝北辰, 张奕华, 赖宜生, 袁胜涛, 张陆勇, 有机化学, 2007, 27, 629.)
[19] Tsang, K. Y.; Sinha, S.; Liu, X.; Bhat, S.; Chandraratna, R. A. US 20050148590, 2005[Chem. Abstr. 2005, 143, 97206].
[20] Nahoum, V.; Pérez, E.; Germain, P.; Rodr??uez-Barrios, F.; Manzo, F.; Kammerer, S.; Lemaire, G.; Hirsch, O.; Royer, C. A.; Gronemeyer, H.; Lera, A. R.; Bourguet, W. Proc. Natl. Acad. Sci. U. S. A. 2007, 104, 17323.
/
| 〈 |
|
〉 |