

Chinese Journal of Organic Chemistry >
Synthesis of Molecular Tweezers Derived from Chenodeoxycholic Acid through Click Reaction and Their Recogniton Properties
Received date: 2014-01-25
Revised date: 2014-03-10
Online published: 2014-04-15
Supported by
Project supported by the Science and Technology Department of Sichuan Province (No. 2012SZ0160) and the National Foreign Expert Bureau (No. 2013-32).
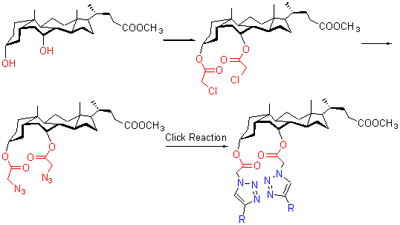
Twelve novel chenodeoxycholic acid-based molecular tweezer metal receptors containing 1,2,3-triazole moieties have been designed and synthesized using click chemistry method. The target compounds were characterized by 1H NMR, IR, MS spectra and elemental analysis. Their binding properties were examined by UV-Vis spectra titration. The results indicate that these molecular tweezers showed high selectivity and affinity for Hg2+ ion.
Zhao Zhigang , Wang Xiaohong , Shi Zhichuan , Cheng Yuyu . Synthesis of Molecular Tweezers Derived from Chenodeoxycholic Acid through Click Reaction and Their Recogniton Properties[J]. Chinese Journal of Organic Chemistry, 2014 , 34(6) : 1110 -1117 . DOI: 10.6023/cjoc201401042
[1] Clarkson. T. W. Am. J. Clin. Nutr. 1995, 61, 682.
[2] Tchounwou, P. B.; Ayensu, W. K.; Ninashvili, N.; Sutton, D.; Environ. Toxicol. 2003, 18, 149.
[3] An, L.; Cai, Y. H.; Yan, C. G. Chin. J. Appl. Chem. 2005, 22, 980 (in Chinese).
(安琳, 蔡亚华, 颜朝国, 应用化学, 2005, 22, 980.)
[4] Silva, A. P. de; Gunaratne, H. Q. N.; Gunnlaugsson, T.; Huxley, A. J. M.; McCoy, C. P.; Rademacher, J. T.; Rice, T. E. Chem. Rev. 1997, 97, 1515.
[5] Kolb, H. C.; Finn, M. G.; Sharpless, K. B. Angew. Chem., Int. Ed. 2001, 40, 2004.
[6] Demko, Z. P.; Sharpless, K. B. Angew. Chem., Int. Ed., 2002, 41, 2110.
[7] Lewis, W. G.; Green, L. G.; Grynszpan, F.; Radic, Z.; Carlier, P. R.; Taylor, P.; Finn, M. G.; Sharpless, K. B. Angew. Chem., Int. Ed. 2002, 41, 1053.
[8] Schweinfurth, D.; Hardcastle, K. L.; Bunz, U. H. F. Chem. Commun. 2008, 1053.
[9] Barreto, A. de F. S.; Vercillo, O. E.; Birkett, M. A.; Caulfield, J. C.; Wessjohann, L. A.; Andrade, C. K. Z. Org. Biomol. Chem. 2011, 9, 5024.
[10] Cecioni, S.; Faure, S.; Darbost, U.; Bonnamour, I.; Parrot-Lopez, H.; Roy, O.; Taillefumier, C.; Wimmerova, M.; Praly, J.-P.; Imberty, A.; Vidal, S. Chem. Eur. J. 2011, 17, 2146.
[11] Maeda, C.; Yamaguchi, S.; Ikeda, C.; Shinokubo, H.; Osaka, A. Org. Lett. 2008, 10, 549.
[12] Wang, J.; He, X.; Gao, L.; Sheng, L.; Shi, X,; Li, J.; Chen, G. Chin. J. Chem. 2011, 29, 1227.
[13] Fazio, M. A.; Lee, O. P.; Schuster, D. I. Org. Lett. 2008, 10, 4979.
[14] Lowe, A. J.; Long, B. M.; Pfeffer, F. M. Chem. Commun. 2013, 49, 3376.
[15] Oh, H.; Han, S. K.; Kim. B. H. Heteroat. Chem. 2012, 23, 187
[16] Hu, J.; Lu, J. R.; Ju, Y. Chem. Asian J. 2011, 6, 2636.
[17] Verzele, D.; Madder, A. Eur. J. Org. Chem. 2013, 673.
[18] Jadhav, J. R.; Bae, C. H.; Kim. H. S. Tetrahedron Lett. 2011, 52, 1623.
[19] Li, W. N.; Xu, Q.; Li, Y.; Zhu, W.; Cui, J. C.; Ju, Y.; Li, G. T. Tetrahedron Lett. 2013, 54, 3868.
[20] Li, D. Z.; Yang, Y. X.; Yang, C.; Hu, B. W.; Huo, B. L.; Xue, L. W.; Wang, A. Z. Tetrahedron 2014, 70, 1223.
[21] Hu, J.; Zhang, M.; Yu, L. B.; Ju. Y. Bioorg. Med. Chem. Lett. 2010, 20, 4342
[22] Kumar, A.; Pandey, P. S. Tetrahedron Lett. 2009, 50, 5842.
[23] Benesi, H. A.; Hildebrand, J. H. J. Am. Chem. Soc. 1949, 71, 2703.
/
| 〈 |
|
〉 |