

Chinese Journal of Organic Chemistry >
Synthesis and Application of pH-Sensitive Linkers in Amphiphilic Block Copolymer
Received date: 2014-03-12
Revised date: 2014-04-28
Online published: 2014-05-07
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 81071250, 91227109), ant the Major Projects in National Science and Technology, “Creation of Major New Drugs” (No. 2011ZX09501-001-05).
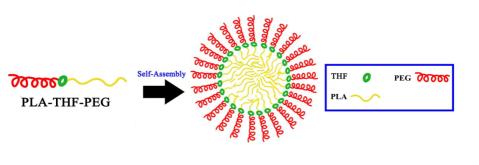
A series of acid cleavable linkers based on tetrahydropyran/tetrahydrofuran (THP/THF) ether were synthesized, in which both of the two terminals are active group which is easy to react with other compounds. The structures for these linkers 1a and 1b were confirmed by 1H NMR, 13C NMR, HRMS, and IR. Diblock cooligomerModel compound containing THP linkage was synthesized. Furthermore a pH-responsive amphiphilic diblock copolymer containing tetrahydrofuran (THF) linkage in which hydrophilic segment was poly(ethylene glycol) (PEG) and hydrophobic segment was hydrophobic polylactic acid (PLA) was synthesized and characterized by 1H NMR, IR and gel permeation chromatography (GPC). The size, stability and critical micelle concentration (CMC) of diblock copolymer micelle were also evaluated. The preliminary experimental results show that the acid sensitive amphiphilic diblock copolymer micelle is a potential alternative for future drug delivery.
Zhu Fangxia , Zhuang Yuan , Li Qing , Yang Qinglai , Wu Xinyan , Shao Zhifen , Gong Bin , Shen Yumei . Synthesis and Application of pH-Sensitive Linkers in Amphiphilic Block Copolymer[J]. Chinese Journal of Organic Chemistry, 2014 , 34(9) : 1806 -1815 . DOI: 10.6023/cjoc201403029
[1] Murthy, N.; Thng, Y.X.; Schuck, S.M.; Xu, C.J.; Fréchet, M.J.J.Am.Chem.Soc.2002, 124, 12398.
[2] Barton, V.; S.Ward, A.; Chadwick, J.; Hill, A.; O'Neill, P.M.J.Med.Chem.2010, 53, 4555.
[3] Olson, E.S.; Jiang, T.; Aguilera, T.A.; Nguyen, Q.T.; Ellies, L.G.; Scadeng, M.; Tsien, R.Y.Proc.Natl.Acad.Sci.U.S.A.2010, 107, 4311.
[4] Louie, A.Y.; Hüber, M.M.; Ahrens, E.T.; Rothbächer, U.; Moats, R.; Jacobs, R.E.; Fraser, S.E.; Meade, T.J.Nat.Biotechnol.2000, 18, 321.
[5] Knapp, D.C.; Serva, S.; D'Onofrio, J.; Keller, A.; Lubys, A.; Kurg, A.; Remm, M.; Engels, J.W.Chem.Eur.J.2011, 17, 2903.
[6] Mura, S, Nicolas, J, Couvreur, P.Nat.Mater.2013, 12, 991.
[7] Cai, X.B.; Hu, W.; Lu, W.Chin.J.Org.Chem.2013, 33, 2520 (in Chinese).(蔡晓冰, 胡薇, 陆国林, 有机化学, 2013, 33, 2520.)
[8] Zhang, Q.; Re Ko, N.; Kwon Oh, J.Chem.Commun.2012, 48, 7542.
[9] Xi, C.B.; Yang, D.; Li, J.; Yan, J.J.; Hu, J.H.Chin.J.Org.Chem.2012, 32, 2166 (in Chinese).(席陈彬, 杨东, 李静, 晏建军, 胡建华, 有机化学, 2012, 32, 2166.)
[10] DiLauro, A.M.; Abbaspourrad, A.; Weitz, D.A.; Phillips, S.T.Macromolecules 2013, 46, 3309.
[11] Tannock, I.F.; Rotin, D.Cancer Res.1989, 49, 4373.
[12] Stubbs, M.; McSheehy, P.M.J.; Griffiths, J.R.; Bashford, C.L.Mol.Med.Today 2000, 6, 15.
[13] Huang, X.Macromolecules 2009, 42, 783.
[14] Zhou, L.; Yu, L.; Ding, M.; Li, J.; Tan, H.; Wang, Z.; Fu, Q.Macromolecules 2011, 44, 857.
[15] Jackson, A.W.; Stakes, C.; Fulton, D.A.Polym.Chem.2011, 2, 2500.
[16] Prabaharan, M.; Grailer, J.J.; Pilla, S.; Steeber, D.A.; Gong, S.Biomaterials 2009, 30, 5757.
[17] Jin, Y.; Song, L.; Su, Y.; Zhu, L.J.; Pang, Y.; Qiu, F.; Tong, G.S.; Yan, D.Y.; Zhu, B.S.; Zhu, X.Y.Biomacromolecules 2011, 12, 3460.
[18] Tanaka, H.; Ishida, T.; Matoba, N.; Tsukamoto, H.; Yamada, H.; Takahashi, T.Chem.Int.Ed.2006, 45, 6349.
[19] Klaikherd, A.; Nagamani, C.; Thayumanavan, S.J.Am.Chem.Soc.2009, 131, 4830.
[20] Gillies, E.R.; Goodwin, A.P.; Fréchet, J.M.J.Bioconjugate Chem.2004, 15, 1254.
[21] Carceller, E.; Merlos, M.; Giral, M.; Bartroli, J.; Garcia-Rafanell, J.; Forn, J.J.Med.Chem.1992, 35, 676.
[22] Beaver, M.G.; Woerpel, K.A.J.Org.Chem.2010, 75, 1107.
[23] (a) Cai, X.; Chorghade, M.S.; Fura, A.; Grewal, G.S.; Jauregui, K.A.; Lounsbury, H.A.; Scannell, R.T.; Yeh, C.G.; Young, M.A.; Yu, S.Org.Process Res.Dev.1999, 3, 73.(b) Pilli, R.A.; Victor, M.M.; de Meijere, A.J.Org.Chem.2000, 65, 5910.(c) Shiro, Y.; Kato, K.; Fujii, M.; Ida, Y.; Akita, H.Tetrahedron 2006, 62, 8687.(d) Hamamoto, H.; Suzuki, Y.; Takahashi, H.; Ikegami, S.Adv.Synth.Catal.2007, 349, 2685.(e) Montagnat, O.D.; Lessene, G.; Hughes, A.B.J.Org.Chem.2009, 75, 390.
[24] Dixon, D.J.; Ley, S.V.; Reynolds, D.J.Chem.Eur.J.2002, 8, 1621.
[25] Nguyen-Ba, N.; Quimpere, M.; Kong, L.C.C.; Brown, W.L.; Dionne, G.US 5789394, 1998 [Chem.Abstr.1998, 129, 161816].
[26] Chow, E.K.-H.; Ho, D.Sci Transl Med 2013, 5(216), 216rv4; Schmalenberg, K.E.; Frauchiger, L.; Nikkhouy-Albers, L.; Uhrich, K.E.Biomacromolecules 2001, 2, 851.
[27] Wang, D.L.; Su, Y.; Zhu, B.S.; Pang, Y.; Zhu, L.J.; Liu, J.Y.; Tu, C.L.; Yan, D.Y.; Zhu, X.Y.Biomacromolecules 2011, 12, 1370.
/
| 〈 |
|
〉 |