

Chinese Journal of Organic Chemistry >
Synthesis of a New Carbohydrate-Derived Chiral Phosphine and Its Coordinating cis- and trans-Pt(II) Complexes
Received date: 2014-04-18
Revised date: 2014-04-28
Online published: 2014-05-07
Supported by
Project supported by the National Natural Science Foundation of China (No. 21272037) and the Natural Science Foundation of Fujian Province (No. 2011J01033).
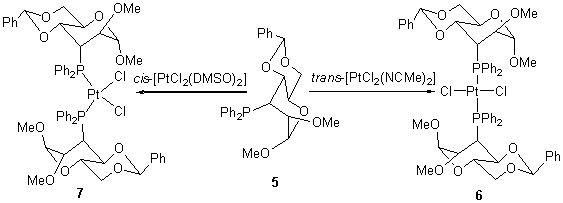
Via four steps of nucleophilic ring-opening of α-D-manno-epoxide with Ph2P- anion, adduction of boranes to phospnine, methylation of hydroxyl, and deprotection of the phosphine-boranes adduct, afforded the desired methyl 3-deoxy-3-diphenylphosphino-2-O-methyl-4,6-O-phenylmethenyl-α-D-pyranoaltroside (5) in 55% of yield. It was found that: (1) the adduct of boranes to the carbohydrate-based phosphine was formed smoothly in the presence of alkyloxide, (2) the alkoxide derived from the secondary alcohol of pyranoside was methylized with MeI as in its sodium salt other than in lithium salt in THF at room temperature, and (3) the deprotection of boranes from the carbohydrate-based phosphine adduct went smoothly by several of organic bases, but more efficiently by ethanol. The corresponding cis- and trans-[Pt(5)2Cl2] formed by the reaction of the carbohydrate-based phosphine 5 with cis- and trans-[Pt(NCMe)2Cl2], respectively. The coupling constant 1JPt-P was reliable to assign the configuration of the geometrical stereoisomers of Pt(II) complexes with the carbohydrate-based phosphine.
Key words: carbohydrate; chiral phosphine; deboronation; Pt(II) complex
Zheng Shan , Jia Li , Liu Zhisen , Jiang Dahong , Huang Yanxian , Nong Nanping , Zhang Qing , Shi Jicheng . Synthesis of a New Carbohydrate-Derived Chiral Phosphine and Its Coordinating cis- and trans-Pt(II) Complexes[J]. Chinese Journal of Organic Chemistry, 2014 , 34(9) : 1840 -1844 . DOI: 10.6023/cjoc201404034
[1] Genet, J.-P.; Ayad, T.; Ratovelomanana-Vidal, V.Chem.Rev.2014, 114, 2824.
[2] Tang, W.; Zheng, X.Chem.Rev.2003, 103, 3029.
[3] Xie, J.-H.; Zhu, S.-F.; Zhou, Q.-L.Chem.Rev.2011, 111, 1713.
[4] Xu, L.; Xia, C.; Sun, W.; Li, F.; Wang, H.Chin.J.Org.Chem.2003, 23, 919 (in Chinese).(徐利文, 夏春谷, 孙伟, 李福伟, 王红旺, 有机化学, 2003, 23, 919.)
[5] Shi, J.-C.; Hong, M.C.; Wu, D.X.; Liu, Q.-T.; Kang, B.-S.Chem.Lett.1995, 685.
[6] Shi, J.-C.; Wen, T.-B.; Wu, D.-X.; Lu, S.-J.; Wang, H.Q.; Kang, B.S.Chin.J.Chem.1996, 14, 454.
[7] Shi, J.-C.; Huang, X.-Y.; Wu, D.-X.; Liu, Q.; Kang, B.Inorg.Chem.1996, 35, 2742.
[8] Shi, J.-C.; Kang, B.-S.; Mak, T.C.W.J.Chem.Soc., Dalton Trans.1997, 2171.
[9] Shi, J.-C.; Chao, H.-Y.; Fu, W.-F.; Chi, C.-M.J.Chem.Soc., Dalton Trans.2000, 3128.
[10] Shi, J.-C.; Yueng, C.-H.; Wu, D.-X.; Liu, Q.; Kang, B.Organometallics 1999, 18, 3796.
[11] Zheng, J.; Zhou, Z.; Tong, Q.; Jiang, F.; Jia, L.; Lin, J.; Shi, J.-C.Chin.J.Org.Chem.2011, 31, 324 (in Chinese).(郑军, 周中高, 童庆松, 江锋,贾莉,林金火,施继成, 有机化学, 2011, 31, 324.)
[12] Shi, J.-C.; Zhou, Z.; Zheng, S.; Zhang, Q.; Jia, L.; Lin, J.Tetrahedron Lett.2014, 55, 2904.
[13] Van Overschelde, M.; Vervecken, E.; Modha, S.G.; Cogen, S.; Van der Eycken, E.; Van der Eycken, J.Tetrahedron 2009, 65, 6410.
[14] Alcock, N.W.; Platt, W.G.; Pringle, P.G.Dalton Trans.1989, 139.
[15] Fanizzi, F.P.; Intini, F.P.; Maresca, L.; Natile, G.Dalton Trans.1990, 199.
[16] Price, J.H.; Williamson, A.N.; Schramm, R.F.; Wayland, B.B.Inorg.Chem.1972, 11, 1280.
/
| 〈 |
|
〉 |