

Chinese Journal of Organic Chemistry >
Synthesis of 1-Chloro-2-aryl Ethyne Using CuCl2·5/3NaCl·5/2Al2O3 as Composite Chlorinating Agent
Received date: 2014-03-15
Revised date: 2014-05-06
Online published: 2014-06-03
Supported by
Project supported by the National Natural Science Foundation of China (No. 21072052) and the Hunan Provincial Science and Technology Department Program (No. 2011 WK4007).
An effective protocol for substitution chlorination was accomplished using a new composite chlorinating agent from CuCl2, inorganic salt and carrier. The most appropriate ratio of each individual component of composite chlorinating agent was determined by comparative experiments. The optimal synthetic conditions for 1-chloro-2-aryl ethyne were obtained by the investigation of various factors, including solvents, the amount of composite chlorinating agents and reaction temperature. The mild and simple method allowed the formation of 1-chloro-2-aryl ethyne in up to 99% yields and showed good selectivities. Various substrates bearing inert or sensitive groups were tolerable for the present preparation. A plausible mechanism for the reaction was also proposed.
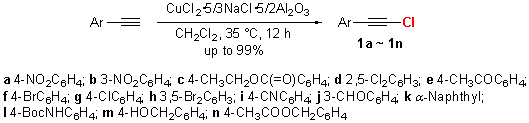
Liu Shuqin , Zhao Zijian , Xiao Jing , Shi Lei , Feng Lingli , Peng Zhihong , An Delie . Synthesis of 1-Chloro-2-aryl Ethyne Using CuCl2·5/3NaCl·5/2Al2O3 as Composite Chlorinating Agent[J]. Chinese Journal of Organic Chemistry, 2014 , 34(10) : 2099 -2105 . DOI: 10.6023/cjoc201403036
[1] (a) Chen, D.-X.; Yuan, Z.-L.; Cai, H.-T.; Zeng, R.-W.; Kong, L.-C.; Zhu, G.-G. J. Org. Chem. 2011, 76, 4071.
(b) Chen, X.-Y.; Chen, D.-X.; Lu, Z.-H.; Kong, L.-C.; Zhu, G.-G. J. Org. Chem. 2011, 76, 6338.
[2] (a) Nishihara, Y.; Ikegashira, K.; Mori, A.; Hiyama, T. Tetrahedron Lett. 1998, 39, 4075.
(b) Nishihara, Y.; Ikegashira, K.; Hirabayashi, K.; Ando, J.-I.; Mori, A.; Hiyama, T. J. Org. Chem. 2000, 65, 1780.
[3] Poulsen, T. B.; Bernardi, L.; Alemán, J.; Overgaard, J.; Jørgensen, K. A. J. Am. Chem. Soc. 2007, 129, 441.
[4] (a) Villeneuve, K.; Riddell, N.; Jordan, R. W.; Tsui, G. C.; Tam, W. Org. Lett. 2004, 6, 4543.
(b) Liu, P.; Jordan, R. W.; Kibbee, S. P.; Goddard, J. D.; Tam, W. J. Org. Chem. 2006, 71, 3793.
(c) Yao, Z.-K.; Yu, Z.-X. J. Am. Chem. Soc. 2011, 133, 10864.
[5] Cahiez, G.; Gager, O.; Buendia, J. Angew. Chem., Int. Ed. 2010, 49, 1278.
[6] Sud, D.; Wigglesworth, T. J.; Branda, N. R. Angew. Chem., Int. Ed. 2007, 46, 8017.
[7] Martins, M. A. P.; Emmerich, D. J.; Pereira, C. M. P.; Cunico, W.; Rossato, M.; Zanatta, N.; Bonacorso, H. G. Tetrahedron Lett. 2004, 45, 4935.
[8] Sasson, Y.; Webster, O. W. J. Chem. Soc., Chem. Commun. 1992, 1200.
[9] Ballester, M.; Castañer, J.; Riera, J.; Tabernero, I. J. Org. Chem. 1986, 51, 1413.
[10] Braga, A. L.; Cornasset, J. V. Synth. Commun. 1989, 19, 2877.
[11] Hashmi, A. S. K.; Ramamurthi, T. D.; Todd, M. H.; Tsang, A. S.-K.; Graf, K. Aust. J. Chem. 2010, 63, 1619.
[12] Vilhelmsen, M. H.; Andersson, A. S.; Nielsen, M. B. Synthesis. 2009, 1469.
[13] Carran, J.; Waschbüsch, R.; Marinetti, A.; Savignac, P. Synthesis 1996, 1494.
[14] Villieras, J.; Perriot, P.; Normant, J. F. Synthesis 1975, 458.
[15] Uemura, S.; Onoe, A.; Okano, M. J. Chem. Soc., Chem. Commun. 1975, 23, 925.
[16] Uemura, S.; Okazaki, H.; Onoe, A.; Okano, M. J. Chem. Soc., Perkin Trans. 1 1977, 676.
[17] Kodomari, M.; Satoh, H.; Yoshitomi, S. Nippon Kagaku Kaishi 1986, 12, 1813.
[18] Bai, D.-H.; Li, C.-J.; Li, J.; Jia, X.-S. Chin. J. Org. Chem. 2012, 32, 994 (in Chinese).
(白东虎, 李春举, 李健, 贾学顺, 有机化学, 2012, 32, 994.)
[19] Jithunsa, M.; Ueda, M.; Miyata, O. Org. Lett. 2011, 13, 518.
[20] Mao, Z.-F.; Wang, Z.; Xu, Z.-Q.; Huang, F.; Yu, Z.-K.; Wang, R. Org. Lett. 2012, 14, 3854.
[21] Wagner, C. D.; Riggs, W. M.; Davis, L. E.; Moulder, J. F. In Handbook of X-ray Photoelectron Spectroscopy, Eds.: Muilenberg, G. E., Perkin-Elmer Press, New York, 1979, p. 82.
[22] Li, S.-P.; Kuang, C.-X.; Sun, Y.-Z. Chem. World 2010, 52 (in Chinese).
(李世鹏, 匡春香, 孙跃枝, 化学世界, 2010, 52.)
[23] Montheard, J.-P.; Camps, M.; Benzaid, A. Tetrahedron Lett. 1982
/
| 〈 |
|
〉 |