

Chinese Journal of Organic Chemistry >
A Study on C—H Carboxylation Reaction of Terminal Alkynes with CO2 in Supercritical CO2
Received date: 2014-03-29
Revised date: 2014-05-16
Online published: 2014-06-03
Supported by
Project supported by the National Natural Science Foundation of China (Nos. 21266019, 21062011, 21362019).
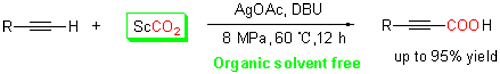
A organic solvent free, new carboxylation pathway for C—H bond of terminal alkynes with supercritical CO2 (ScCO2) has been developed by using Ag(I)/DBU (1,8-diazabicyclo(5.4.0)undec-7-ene) catalytic system to obtain propiolic acid products with excellent yields in this work. In our reaction system, ScCO2 not only acts as reactive solvent but also as reactant. DBU plays the roles of co-catalyst, nucleophile and base, and obviously enhances the reaction rate in ScCO2. The Ag(I)/DBU catalytic system exhibits higher activity and wide substrate scope. Notably, both liquid and solid terminal alkynes can smoothly react with ScCO2 to produce desired product. The reaction pathway of functionalized propiolic acid formation from the carboxylation of terminal alkynes with CO2 is environment-friendly, simple, and economic.
Key words: AgOAc; DBU; terminal alkyne; ScCO2; carboxylation
Li Fawang , Suo Quanling , Hong Hailong , Zhu Ning , Wang Yaqi , Han Limin . A Study on C—H Carboxylation Reaction of Terminal Alkynes with CO2 in Supercritical CO2[J]. Chinese Journal of Organic Chemistry, 2014 , 34(10) : 2172 -2177 . DOI: 10.6023/cjoc201403064
[1] (a) Lehmann, F.; Lake, L.; Currier, E. A.; Olsson, R.; Hacksell, U.; Luthman K. Eur. J. Med. Chem. 2007, 42, 276.
(b) Ho, Y.-S.; Duh, J.-S.; Jeng, J.-H.; Wang, Y.-J.; Liang, Y.-C.; Lin, C.-H.; Tseng, C.-J.; Yu, C.-F.; Chen, R.-J.; Lin, J.-K. Int. J. Cancer 2001, 91, 393.
(c) Dong, Y.; Guo, X.-Y.; Yu, Y.-Y.; Liu, G. Mol. Diversity 2013, 17, 1.
(d) Jiang, Y.-B.; Han, C.-M.; Liang, X.-Q.; Yang, P.; Wang, H. Chin. J. Org. Chem. 2012, 32, 1884 (in Chinese).
(江玉波, 韩春美, 梁雪秋, 杨朋, 王红, 有机化学, 2012, 32, 1884.)
[2] (a) Moon, J.; Jeong, M.; Nam, H.; Ju, J.; Moon, J. H.; Jung, H. M.; Lee, S. Org. Lett. 2008, 10, 945.
(b) Li, Y.; Jardine, K. J.; Tan, R.; Song, D.; Dong, V. M. Angew. Chem., Int. Ed. 2009, 121, 9870.
(c) Bararjanian, M.; Balalaie, S.; Rominger, F.; Movassagh, B.; Bijanzadeh, H. R. J. Org. Chem. 2010, 75, 2806.
(d) Feng, H.; Ermolat'ev, D. S.; Song, G.; Van der Eycken, E. V. Adv. Synth. Catal. 2012, 354, 505.
(e) He, Z.-B.; Zhang, R.; Hu, M.-Y.; Li, L.-C.; Ni, C.-F.; Hu, J.-B. Chem. Sci. 2013, 4, 3478.
[3] Arndt, M.; Risto, E.; Krause, T.; Gooßen, L. J. ChemCatChem 2012, 4, 484.
[4] (a) Omae, I. Coord. Chem. Rev. 2012, 256, 1384.
(b) Cokoja, M.; Bruckmeier, C.; Rieger, B.; Herrmann, W. A.; Kühn, F. E. Angew. Chem., Int. Ed. 2011, 50, 8510.
(c) Tsuji, Y.; Fujihara, T. Chem. Commun. 2012, 48, 9956.
(d) Yang, Z.-Z.; He, L.-N.; Gao, J.; Liu, A.-H.; Yu, B. Energy Environ. Sci. 2012, 5, 6602.
(f) Huang, K.; Sun, C.-L.; Shi, Z.-J. Chem. Soc. Rev. 2011, 40, 2435.
[5] (a) Sakakura, T.; Choi, J.-C.; Yasuda, H. Chem. Rev. 2007, 107, 2365.
(b) Sakakura, T.; Kohon, K. Chem. Commun. 2009, 1312.
(c) Mikkelsen, M.; Jørgensena, M.; Krebs, F. C. Energy Environ. Sci. 2010, 3, 43.
(d) Huang, K.; Sun, C.-L.; Shi, Z.-J. Chem. Soc. Rev. 2011, 40, 2435.
(e) Izumi, Y. Coord. Chem. Rev. 2013, 257, 171.
(f) Wang, H.-Y.; Xu, J.-F.; Jing, H.; Zhang, J.; Li, P.-Q.; Lu, F.-S. Acta Chim. Sinica 2013, 71, 941 (in Chinese).
(王祜英, 徐金凤, 井华, 张军, 李培强, 路福绥, 化学学报, 2013, 71, 941.)
[6] (a) Shi, M.; Nicholas, M. J. Am. Chem. Soc. 1997, 119, 5057.
(b) Johansson, R.; Wendt, O. F. Dalton Trans. 2007, 488.
(c) Wu, J.-G.; Hazari, N. Chem. Commun. 2011, 47, 1069.
[7] (a) Yeung, C. S.; Dong, V. M. J. Am. Chem. Soc. 2008, 130, 7826.
(b) Ochiai, H.; Jang, M.; Hirano, K.; Yorimitsu, H.; Oshima, K. Org. Lett. 2008, 10, 2681.
(c) Kobayashi, K.; Kondo, Y. Org. Lett. 2009, 11, 2035.
(d) Correa, A.; Martín, R. Angew. Chem., Int. Ed. 2009, 48, 6201.
[8] (a) Ohishi, T.; Nishiura, M.; Hou, Z. Angew. Chem., Int. Ed. 2008, 47, 5792.
(b) Takaya, J.; Tadami, S.; Ukai, K.; Iwasawa, N. Org. Lett. 2008, 10, 2697.
(c) Ukai, K.; Aoki, M.; Takaya, J.; Iwasawa. N. J. Am. Chem. Soc. 2006, 128, 8706.
(d) Zhang, X.; Zhang, W.-Z.; Shi, L.-L.; Guo, C.-X.; Zhang, L.-L.; Lu, X.-B. Chem. Commun. 2012, 48, 6292.
(e) Wang, W.-L; Zhang, G.-D.; Lang, R.; Xia, C.-G.; Li, F.-W. Green Chem. 2013, 15, 635.
[9] (a) Sasano, K.; Takaya, J.; Iwasawa, N. J. Am. Chem. Soc. 2013, 135, 10954.
(b) Zhang, L.; Hou, Z.-M. Pure Appl. Chem. 2012, 84, 1705.
(c) Ackermann. L. Angew. Chem., Int. Ed. 2011, 50, 3842.
(d) Boogaerts, I. I. F.; Nolan, S. P. J. Am. Chem. Soc. 2010, 132, 8858.
(e) Boogaerts, I. I. F.; Fortman, G. C.; Furst, M. R. L.; Cazin, C. S. J.; Nolan, S. P. Angew. Chem., Int. Ed. 2010, 49, 8674.
(f) Zhang, L.; Cheng, J.; Ohishi, T.; Hou. Z. Angew. Chem. Int. Ed. 2010, 49, 8670.
(g) Inomata, H.; Ogata, K.; Fukuzawa, S. I.; Hou Z. Org. Lett. 2012, 14, 3986.
(h) Vechorkin, O.; Hirt, N.; Hu, X. Org. Lett. 2010, 12, 3567.
[10] Manjolinho, F.; Arndt, M.; Gooßen, K.; Gooßen, L. J. ACS Catal. 2012, 2, 2014.
[11] (a) Yu, B.; Diao, Z.-F.; Guo, C.-X.; Zhong, C.-L.; He, L.-N.; Zhao, Y.-N.; Song, Q.-W.; Liu, A.-H.; Wang, J.-Q. Green Chem. 2013, 15, 2401.
(b) Inamoto, K.; Asano, N.; Kobayashi, K.; Yonemoto, M.; Kondo, Y. Org. Biomol. Chem. 2012, 10, 1514.
(c) Zhang, W.-Z.; Li, W.-J.; Zhang, X.; Zhou, H.; Lu, X. B. Org. Lett. 2010, 12, 4748.
(d) Oi, S.; Fukue, Y.; Nemoto, K.; Inoue, Y. Macromolecules 1996, 29, 2694.
(e) Fukue, Y.; Oi, S.; Inoue, Y. J. Chem. Soc., Chem. Commun. 1994, 2091.
[12] Gooßen, L. J.; Rodrguez, N.; Manjolinho, F.; Lange, P. P. Adv. Synth. Catal. 2010, 352, 2913.
[13] Yu, D.; Zhang, Y. Proc. Natl. Acad. Sci. U. S. A.2010, 107, 20184.
[14] Yu, D.; Tan, M. X.; Zhang, Y. Adv. Synth. Catal. 2012, 354, 969.
[15] Yu, D.; Zhang, Y. Green Chem. 2011, 13, 1275.
[16] Ouyang, K.-B.; Xi, Z.-F. Acta Chim. Sinica 2013, 71, 13 (in Chinese).
(欧阳昆冰, 席振峰, 化学学报, 2013, 71, 13.)
[17] (a) Zhang, Y.-P.; Bian, M.; Yao, W.-J.; Gu, J.-M.; Ma, C. Chem. Commun. 2009, 4729.
(b) Qi, C.; Jiang, H.; Huang, L.; Yuan, G.; Ren, Y. Org. Lett. 2011, 13, 5520.
(c) Zheng, D.-Q.; Li, S.-Y.; Wu, J. Org. Lett. 2012, 14, 2655.
(d) Li, Z.-G.; Sun, H.-B.; Jiang, H.-L.; Liu, H. Org. Lett. 2008, 10, 3263.
[18] (a) Yoshida, M.; Komatsuzaki, Y.; Ihara, M. Org. Lett. 2008, 10, 2083.
(b) Heldebrant, D. J.; Yonker, C. R.; Jessop, P. G.; Phan, L. Energy Environ. Sci. 2008, 1, 487.
(c) Dinsmore, C. J.; Mercer, S. P. Org. Lett. 2004, 6, 2885.
[19] Yoshida, M.; Mizuguchi, T.; Shishido, K. Chem. Eur. J. 2012, 18, 15578.
[20] Li, J.-H.; Jia, L.-Q.; Jiang, H.-F. Chin. J. Org. Chem. 2000, 20, 293 (in Chinese).
(李金恒, 贾兰齐, 江焕峰, 有机化学, 2000, 20, 293.)
[21] Ma, L.; Dolphin, D. J. Chem. Soc., Chem. Commun. 1995, 2251.
[22] (a) Wang, Y.; Han, Q.-Z.; Wen, H. Mol. Simul. 2013, 39, 822.
(b) Heldebrant, D. J.; Jessop, P. G.; Thomas, C. A.; Eckert, C. A.; Liotta, C. L. J. Org. Chem. 2005, 70, 5335.
(c) Endo, T.; Nagai, D.; Monma, T.; Yamaguchi, H.; Ochiai, B. Macromolecules 2004, 37, 2007.
[23] Wang, X.; Lim, Y. N.; Lee, C.; Jang, H.-Y.; Lee, B. Y. Eur. J. Org. Chem. 2013, 1867.
/
| 〈 |
|
〉 |